Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias geológicas
versión On-line ISSN 2007-2902versión impresa ISSN 1026-8774
Rev. mex. cienc. geol vol.26 no.3 Ciudad de México dic. 2009
Colimaite, K3VS4 – a new potassium–vanadium sulfide mineral from the Colima volcano, State of Colima (Mexico)
Colimaíta K3VS4 – un nuevo mineral de potasio y vanadio del grupo de los sulfuros proveniente del volcán de Colima, estado de Colima (México)
Mikhail Ostrooumov1 *, Yuri Taran 2, María Arellano–Jiménez 3, Alfredo Ponce3, and José Reyes–Gasga3
1 Universidad Michoacana de San Nicolás de Hidalgo, Instituto de Investigaciones Metalúrgicas, Departamento de Mineralogía, Ciudad Universitaria, Edificio U, 58000 Morelia, Michoacán, Mexico.*ostroum@zeus.umich.mx
2 Universidad Nacional Autónoma de México, Instituto de Geofísica, Ciudad Universitaria, 04510 Mexico, D. F., Mexico.
3 Universidad Nacional Autónoma de México, Instituto de Física, Departmento de Materia Condensada, A. P. 20–364, 01000 Mexico D. F., Mexico.
Manuscript recieved: December 2, 2008
Corrected manuscript received: June 9, 2009
Manuscript accepted: June 11, 2009
ABSTRACT
Colimaite, K3VS4, has been discovered in the active fumaroles of the Colima volcano crater, Mexico. The mineral is named colimaite after the locality, which, at the same time, is the current active volcanic crater and the name of the State of Colima (Mexico). Colimaite is the naturally occurring analog of synthetic K3VS4. The mineral formed as a sublimate from the volcanic gases and is associated with cristobalite, arcanite, thenardite, barite and native gold. Colimaite occurs in "hedgehog"–like particles, which contain the needle crystals, up to 50 µm length and 20 µm width. Electron microprobe analyses gave S=43.29 %, K=39.36 %, V=17.41 %, Na=0.43 %, with the sum of 100.49 (wt.%), as the mean of six measurements, previously tested as discordant outlier–free statistical samples. The empirical formula, calculated on the basis of eight atoms, is (K2.95Na0.06) ∑3.01V1.03 S3.97. The idealized formula is K3VS4. The Selected Area Electron Diffraction (SAED) and X–ray powder diffraction data (Cu Kα radiation) indicated that the structure of the micro–sized particles correspond to the orthorhombic K3VS4 crystalline phase: space group Pnma, with a=9.139 (5), b=10.625 (7), c=9.135 (3) Å, V=887.03 (9) Å3, and Z=4. The five strongest calculated diffraction lines from this natural compound are [d in Å, (I) (hkl)]: 2.806 (100)(230), 3.463 (73)(220), 2.785 (70)( 113), 2.928 (67)(013), and 2.677 (63)(132). SAED patterns are quite similar to those of the synthetic K3VS4. The calculated density (Z=4) is 2.235 g cm–3. The main observed Raman bands lie in the region below 500 cm–1 and the most characteristic bands occur between 150 and 300 cm–1: 192, 203, 245, 264, 277 and 297 cm–1. Colimaite, K3VS4, is the first newly recognized mineral species collected from an active fumarole in this volcanic crater. The mineral and the mineral name have been approved by the Commission on New Minerals, Nomenclature and Classification (CNMNC) of the International Mineralogical Association (IMA # 2007–045).
Key words: colimaite, sulfide, new mineral, Colima volcano, Mexico.
RESUMEN
La colimaíta, K3VS4, es un nuevo mineral recientemente descubierto en las fumarolas del cráter del volcán de Colima, México. La colimaíta es el análogo natural del K3VS4 sintético y se encontró entre los sublimados de gases volcánicos en paragénesis con cristobalita, arcanita, tenardita y oro nativo. Es un mineral de color verde amarillento, con lustre no metálico, que forma cristales aciculares de hasta 50 µm de largo y 20 µm de ancho. A partir de análisis por microsonda electrónica se obtuvo la composición S=43.29 %, K=39.36 %, V=17.41 %, Na=0.43 %, con una suma de 100.49 % en peso, como la media de seis mediciones previamente probadas como libres de valores discordantes. La fórmula cristaloquímica, calculada con base en ocho átomos, es la siguiente: (K2.95Na0.06) ∑3.01V1.03 S3.97 , y la fórmula idealizada es K3VS4 . Datos de Difracción electrónica de Área Selecta y de difracción de rayos X (Cu Kα) indicaron que la colimaíta pertenece al sistema rómbico (grupo espacial Pnma) con los siguientes parámetros de la celda elemental: a=9.139 (5) Å, b=10.625 (7) Å, c=9.135 (3) Å, V=887.03 (9) Å3 y Z=4. Las cinco líneas más intensas de difracción de rayos X [d (Å), (I), (hkl)] son 2.806 (100)(230), 3.463 (73)(220), 2.785 (70)(113), 2.928 (67)(013) y 2.677 (63)(132). Los patrones de Difracción de Área Selecta son similares a los de la fase sintética K3VS4. La densidad calculada (Z=4) es 2.235 g cm–3. Las bandas principales en el espectro Raman se localizan en la región de bajas frecuencias a menos de 500 cm–1 y las bandas más características se encuentran entre 150 y 300 cm–1: 192, 203, 245, 264, 277 y 297 cm–1. La colimaíta, K3VS4, es una nueva especie mineral, descrita aquí por primera vez, que se encuentra asociada a diversos minerales ya conocidos en las fumarolas del volcán de Colima. La colimaíta y su nombre han sido aprobados por la "Commission on New Minerals, Nomenclature and Classification" (CNMNC) de la "International Mineralogical Association" (IMA) con el voto No. # 2007–045.
Palabras clave: colimaíta, sulfuro, nuevo mineral, volcán Colima, México.
INTRODUCTION
This paper presents data regarding the discovery of potassium–vanadium sulfide on the inner wall of 1 m–long silica tubes inserted into a 800 °C fumarolic vent in the crater of Colima volcano, Mexico (Ostrooumov et al., 2008). The mineral is named colimaite after the locality that, at the same time, is the current active volcanic crater and the name of the State of Colima (Mexico). Colimaite, K3VS4, is the first newly recognized mineral species collected from an active fumarole in this volcanic crater, which is the most active volcano of Mexico and one of the most active in the Americas. Moreover, this is the first new mineral species discovered in Mexico after 1998 (Ostrooumov, 2001).
The mineral and the mineral name have been approved by the Commission on New Minerals, Nomenclature and Classification (CNMNC) of the International Mineralogical Association (IMA# 2007–045). The holotype sample has been deposited in the Mexican new mineral collection of the Sociedad Mexicana de Mineralogía (Facultad de Ingeniería, Universidad Nacional Autónoma de México) with No. FIM 08/01.
OCCURENCE
Colima volcano (19°30'45''N, 103°37'W, 3855 m above sea level) is a Quaternary andesite volcano that has been intermittently active during the modern eruptive history (1930–1994). This volcano is located in the western portion of the Trans–Mexican Volcanic Belt (on the border between Colima and Jalisco States, Mexico) and its geology and erupted products have been described in detail by Luhr and Carmichael (1990). The crater of the Colima volcano can be divided into several zones, characterized by different gas discharge temperatures (Connors at al., 1993). The zone with the highest temperature (Z3) is located in the northern part of the crater (Figure 1). Red–glowing holes with temperatures of 700–800 °C can be seen in this area between lava blocks covered by yellow–green and green–blue incrustations. Yellow and white–yellow, sulfur–like incrustations, often forming small stalactites in cracks and niches, occur in the intermediate–temperature (~400 °C) zone Z2.
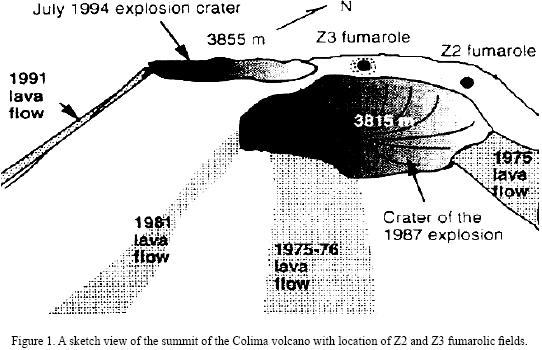
The fumaroles of Colima volcano have been a rich source of different mineral assemblages, which have been described by Taran et al. (2000, 2001). These mineral aggregates have been formed in different fumarolic fields into several crater zones characterized by the highest (800 °C) and middle–temperatures (400 °C). The important feature of the Colima mineral precipitates is, so far, the presence of mixed or pure Na–K sulfates enriched in V, Zn, Pb and Cu, and a complete lack of sulfide and minerals containing Mo and Cd.
EXPERIMENTAL PROCEDURE
Experiments with silica tubes inserted into vents to recover samples were conducted at the Colima volcano twice in 1996. Two 1–m–long tubes were inserted into the high–temperature vent at site Z3. The first tube, Colima 1, with a diameter of 20 mm, was left for two weeks. The second one, Colima 2, with a diameter of 35 mm, remained in place for 80 days. From the beginning of the experiments, the only vent temperatures were measured at 1 m depth (765 °C and 801°C, respectively). The temperature distribution inside the tube was measured with a thermocouple at the completion of the experiment; the temperature gradient in the narrow tube was 780–350 °C, and in the wide tube, 828–380 °C. The temperature of the gases at the sampling site was about 400–800 °C.
In the laboratory, each tube was cut into ten pieces corresponding to ten temperature zones (1–2: 380–420 °C; 3: 450 °C; 4: 550 °C; 5–6: 600 °C; 7: 680 °C; 8: 740°C; 9–10: 828 °C), and mineral precipitates were studied and analyzed by different analytical methods.
Two different microscopes were used to observe the samples: a low–vacuum scanning electron microscope (LV–SEM) Jeol 5600LV, and a transmission electron microscope (TEM) Jeol 100CX. The LV–SEM has attached an X–ray energy dispersive spectroscopy (EDS) equipment NORAN–EDS for chemical analysis of the samples. The sample was crushed and deposited on a cooper grid for the analysis in the JEOL 100 CX transmission electron microscope operated at 100 kV.
Selected area electron diffraction (SAED) patterns were obtained in a rotation–double–tilt holder and registered on standard photographic films. The electron diffraction patterns images were digitalized and calibrated for the indexing. The composition of colimaite was determined by wavelength dispersive spectroscopy (WDS) using a JEOL JXA–8900R (scanning electron microscope–electron microprobe) operating at 20 kV and 1–2 nA, with a beam diameter of ~2 µm. In this study, three crystals were examined with a Raman microprobe (RMP).
The Raman spectra were recorded using the λL=514.5 nm line of an Ar+ laser with a Jobin–Yvon T64000 spectrometer equipped with a multichannel charge–coupled device (CCD) detector cooled at 77 K. The samples were analyzed under an Olympus microscope with 50x and 100x (times) objectives giving a 2 micrometer spatial resolution in confocal setting. The spectral slit width was 2–2.5 cm–1, yielding an experimental spectral resolution of ± 1 cm–1. X–ray diffraction (XRD) analyses were performed with a Brüker AXS–D8 Advanced diffractometer and a Brüker D8 Discover diffractometer with General Area Detector Diffraction System V4.1.27 (GADDS), both instruments with Cu–Kα monochromatic radiation.
MORPHOLOGY AND PHYSICAL PROPERTIES
Colimaite was found in a narrow temperature interval of 450–600 °C (zones 3–5, see below) in both sampling silica tubes (Colima 1 and Colima 2) (Ostrooumov and Taran, 2001). This potassium sulfide forms aggregates of yellow–green micro–sized needles (10–100 µm in size) and crystallized in association with cristobalite, arcanite, thenardite, baryte and native gold.
The particles of colimaite were found on a very thin layer on a glassy–like thicker support, which is indicated by an arrow in the cross–sectional, scanning electron microscope (SEM) image showed in Figure 2. The thickness of this film is of approximately 25 µm. EDS analyses of this sample along the cross–sectional direction indicated that the glassy–like support (the bright zone in Figure 2a) has a composition that corresponds to a silicon oxide. The darker zone corresponds to the carbon paint used to glue the sample to the SEM holder.
The samples contain a huge number (approximately 4.7 x 103 particles cm–2; see Figure 3) of micrometric particles surrounded by thorns which are named "hedgehog"–like particles hereafter. Colimaite occurs as extremely fine needles (a few µm thick) intergrown in small "hedgehog"–like aggregates (about 100 µm in size). These "hedgehog"–like particles contained the crystals with acicular habit, up to 50 µm length and 20 µm width. The crystallographic forms of these crystals were not observed.

To get better information on the surface morphology of the particles, it is recommended to obtain SEM images at lower accelerating voltage, such as those shown in Figure 4a. It is worth noting the particle indicated by the arrow, because it is partially covered with a small thorny area (Figures 4b, 4c). The 5 kV images allow also getting more information of the thorny–like aspect of these particles and of the existence of smaller regular and irregular particles among these thorns. In the case of the thorns, the 5 kV images allowed to discover that they are in fact composed by many small and regular particles, such as those observed in Figure 4d. These regular particles are of two types: regular parallelepipeds and elongated rectangular prisms.
Colimaite is opaque to transmitted light; in reflected light in the air, the mineral has a dark golden color and is nonpleochroic. Streak: yellow–green. Luster: resinous to greasy. Non–fluorescent. Hardness (H) could not be measured. Tenacity: brittle. Cleavage: none observed. Fracture: splintery. Density could not be measured because of small grain size. Density (calc.) = 2.235 g cm–3. Optical properties in incident light and reflectance could not be determined because of the extremely small grain size.
CHEMICAL COMPOSITION
Preliminary qualitative EDS analyses from the "hedgehog"–like particles showed that of the elements with Z > 8, only K, V, S, Na and Si were present above background. Many EDS spectra were obtained and EDS chemical mapping was performed not only on the "hedgehog"–like particles but also on their environs (Figure 5f). These maps also indicate the existence of V, K, Na, and S in the "hedgehog"–like particles (Figure 5a, 5c–5e). Important to note is that the presence of Si (Figure 4b) corresponds to the material from the silica tube matrix.
Subsequent quantitative analyses by WDS were done on six different points of the "hedgehog"–like particles using well analyzed calibration standards (Table 1). These data give relevant information on the composition of the "hedgehog"–like particles, although some variation in the element percentage between the analyzed sites is present. Mean and standard deviation values for colimaite were estimated (Table 1) for six electron–microprobe analyses after carefully testing the measured data for possible discordant outliers using the unpublished computer program DODESYS by S.P. Verma and L. Díaz–González, which uses new precise and accurate critical values (Verma and Quiroz–Ruiz 2008; Verma et al. 2008). No discordant outliers were observed ascertaining the good quality of the chemical analyses and the use of statistically normal samples for the calculation of chemical formula. WDS analyses were carried out inside the thorns shown in Figure 4 and the empirical formula, calculated on the basis of eight atoms, is (K2.45, Na0.06)∑3.01V1.03S3.97. The chemical formula of the analyzed mineral was also calculated as (K2.96, Na0.06)V1.01S3.97 from the precise atomic weights of the International Union of Pure and Applied Chemistry (IUPAC) (Vocke, 1997). Thus, the simplified formula is K3VS4.
X–RAY CRYSTALLOGRAPHY AND SAED DATA
Single–crystal X–ray study was impossible because of the extremely small size of the crystals and their sticking to one another. An X–ray study of the colimaite was carried out by powder techniques. The X–ray powder diffraction data are presented in Table 2. The experimental XRD patterns have been indexed using the international JCPDF (Joint Committee for Powder Diffraction Files) database, searchable by the position of the X–ray diffraction peaks. All peaks of this diffractogram were easily indexed as K3VS4.
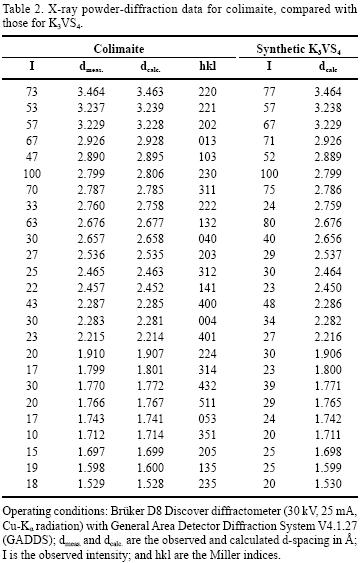
The five strongest lines (Table 2) of this difractogram [d in Å, (I ) (hkl )] appearing in X–ray powder diffraction patterns (Cu Kα radiation) were: 2.806 (100)(230), 3.463 (73)(220), 2.785 (70)(113), 2.928 (67)(013), and 2.677 (63)(132). The IND and PARAM PDWin software programs (NPP Burevestnik, St.Petersburg, Russia) were used for the determination of the unit cell dimensions. Analysis of the X–ray powder pattern (Table 2) demonstrates that colimaite is a natural analogue of the well–known synthetic compound K3VS4 (Duerichen and Bensch 1996). The X–ray powder pattern of K3VS4 was indexed in orthorhombic symmetry, space group Pnma (62), and the refined unit–cell parameters were refined from 37 powder–diffraction reflections, representing d values between 3.5 and 1.1 Å, for which unambiguous indexing was possible on the basis of an analogy with the synthetic equivalent. Comparable powder diffraction data for the synthetic equivalent is presented in the Powder Diffraction File: 86–0712 (calculated). The unit cell parameters determined with the above method were as follows: a= 9.139 (5) Å, b= 10.625 (7) Å, c= 9.135 (3) Å, V=887.03 (9) Å3, Z=4. The a:b:c ratio calculated from the unit–cell parameters was: 0.8601:1:0.8598. Therefore, the structure of the "hedgehog"–like particles is related with the K3VS4 compound.
The TEM analysis and the selected area electron diffraction patterns (SAED) of the sample supports the last asseveration. Figures 6a and 6b show the bright field image and selected area electron diffraction patterns from the thorns of the "hedgehog" particles. Some of these diffractions patterns could be indexed as SiO2 – cristobalite, and as K3VS4 (orthorhombic).
The electron diffraction patterns images were digitalized and calibrated for indexing. Figure 6c shows SAED patterns from a polycrystalline region of the sample with the "hedgehog" particles. Also, the radial intensity was obtained from the SAED pattern using the process diffraction software package. The inverse of the distances measured from the centre of the SAED patterns correspond to the interplanar spacing of the polycrystalline region. The experimental interplanar spacing measured from the rings in the SAED patterns correspond to the peaks in the graph shown in Figure 6c. These peaks correlate with the interplanar spacing of the K3VS4 crystalline phase.
Duerichen and Bensch (1996) reported crystal structure data for the synthetic equivalent of the colimaite. The crystal structure of colimaite, as well as its synthetic analogue K3VS4, is built up by vanadium centered sulfur tetrahedra separated by potassium ions. The V–S distances vary between 2.137 Å and 2.163 Å and are in the range reported for VS4 tetrahedra–containing compounds. In K3VS4, the K(1) ion is surrounded by five sulfur atoms with K(1)–S ranging from 3.130 Å to 3.384 Å and an average of 3.296 Å. Two further atoms are at a distance of 3.771 Å, which seems to be too large for significant bonding interactions. The K(2) ion is bound to eight sulfur atoms and the K(2)–S bond lengths vary from 3.176 Å to 3.495 Å. The average K(2)–S distance is 3.314 Å. Both K+ ions are in an irregular coordination polyhedron.
RAMAN SPECTROMETRY
RMP provides information that is difficult to obtain from other widely used techniques such as electron microprobe and ion microprobe. These latter techniques can readily identify, map out the distribution and determine the quantity of the elemental constituents, but they can not identify the type of chemical bonding between atoms present as specific compounds in a microsample. The unpolarized room–temperature Raman spectra of colimaite is reported for the first time (Figure 7) and tentative assignments have been proposed for the main Raman bands, based on comparisons with other sulfide spectra. The frequencies of the Raman bands observed in all the samples used in this study are summarized in Table 3, as well as the spectra of other vanadium sulfides (Downs, 2006) included for comparison. The resolution of the Raman bands obtained in this study was around 0.5 to 1.0 cm–1.
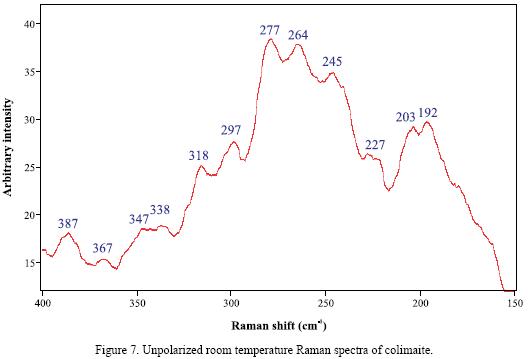
In the Raman spectrum of colimaite (Figure 7), it can be readily seen that the strongest bands fall in two definitely limited frequency regions, which are centered around 277 and 192 cm–1, respectively. Taking into account the frequencies observed in the Raman spectra of some vanadium sulfides (Table 3), it can be inferred that the main observed bands of these two frequency groups, 277–245 and 203–192 cm–1, contain the V–S stretching (A1) and bending (B1) modes, respectively (Nakamoto, 1978). A more reliable assignment in this case could be established through the comparison with the spectra of other minerals or compounds possesing an analogous structure.
CONCLUSIONS
This paper reports the first finding of K3VS4, named colimaite, precipitated from high–temperature (450–600 °C) volcanic vapor in silica tube experiments. Colimaite is another vanadium mineral discovered in volcanic fumaroles. The vanadium mineralization is common in fumarolic environments and has been observed at a number of localities all over the world (Vergasova and Filatov, 1993). However, colimaite is the only potassium vanadium sulfide found in fumaroles so far. This is the first finding of potasium vanadium sulfide in silica tube experiments at volcano sites. Colimaite precipitates from the high–temperature vapor of Colima volcano in the form of "hedgehog"–like particles, with needle crystals up to 50 µm length and 20 µm width, in association with cristobalite, arcanite, thenardite, barite and native gold. This sulfide mineral phase is orthorhombic (space group Pnma) with a= 9.139 Å, b= 10.625 Å and c= 9.135 Å. TEM–SAED, SEM–EDS, XRD, and RMP data confirmed that colimaite is new to the mineralogical science. The mineral and the mineral name have been approved by the Commission on New Minerals, Nomenclature and Classification (CNMNC) of the International Mineralogical Association (IMA# 2007–045).
ACKNOWLEDGEMENTS
The authors wish to thank M.C. Ellen Graber (Instituto Tecnológico de Monterrey) for the English revision of this work. The critical reviews of two referees (Dr. Eugene Choumiline and Dr. George Harlow) are gratefully acknowledged. The authors express their warmest thanks to editor in chief, Dr. Surendra P. Verma for his helpful suggestions, which greatly improved the manuscript.
REFERENCES
Connor, C.B., Clement, B.M., Song, X., Lane, S.B., West Thomas, J., 1993, Continuous monitoring of high–temperatures fumaroles on an active lava dome, volcan Colima, Mexico. Evidence of mass flow variation in response to atmospheric forcing: Journal of Geophysical Research, 98(B11), 19713–19722. [ Links ]
Downs, R.T., 2006, The RRUFF project: an integrated study of the chemistry, crystallography, Raman and infrared spectroscopy of minerals, in Program and Abstracts of the 19th General Meeting of the International Mineralogical Association, Kobe, Japan, O03–13, http://rruff.info/index.php. [ Links ]
Duerichen, P., Bensch, W., 1996, Synthesis and crystal structures of K3VS4 and K2CuVS4: first examples of ternary and quaternary vanadium sulfides prepared via the molten flux method: European Journal Solid State, Inorganic Chemistry, 33(4), 309–320. [ Links ]
Luhr, J.F., Carmichael, I.S.E., 1990, Geology of volcán de Colima: Universidad Nacional Autónoma de México, Instituto de Geología, 107, 101 pp. [ Links ]
Nakamoto, K., 1978, Infrared and Raman spectra of Inorganic and Coordination Compounds: New York, John Willey & Sons, 338 pp. [ Links ]
Ostrooumov, M., 2001, Mineralogía Avanzada en México: conceptos, resultados, investigaciones futuras (on line): Boletín de la Sociedad Mexicana de Mineralogía, 14, 7–16, http://www.iim.umich.mx/smexmineralogia/holotipos.htm. [ Links ]
Ostrooumov, M., Taran, Y.A., 2001, Paragénesis de las formaciones minerales recientes: minerales sublimados en el volcán Colima, México: Boletín de la Sociedad Mexicana de Mineralogía, 14, 38–39. [ Links ]
Ostrooumov, M., Taran Y.A., Arellano–Jimenez, M., Ponce, A., Reyes–Gasga, J., 2008, La colimaíta, K3VS4, un nuevo mineral del volcán Colima (México): Boletín de la Sociedad Mexicana de Mineralogía, 18, 7–8. [ Links ]
Taran, Y.A., Bernard, A., Gavilanes, J.C., Africano, F., 2000, Native gold in mineral precipitates from high–temperature volcanic gases of Colima volcano, México: Applied Geochemistry, 15(3), 337–346. [ Links ]
Taran, Y.A., Bernard, A., Gavilanes, J.C., Lounezheva, E., Cortés, A., Armienta, M.A., 2001, Chemistry and mineralogy of high–temperature gas discharges from Colima volcano, México. Implications for the magmatic gas–atmosphere interaction: Journal of Volcanology and Geothermal Research, 108(1–4), 245–264. [ Links ]
Vergasova, S., Filatov, S., 1993, Minerals of volcanic exhalations–a new genetic group (after the data of Tolbachik volcano eruption in 1975–1976): Proceedings of the Russian Mineralogical Society, 122, 68–76. [ Links ]
Verma, S.P., Quiroz–Ruiz, A., 2008, Critical values for 33 discordancy test variants for outliers in normal samples of very large sizes from 1,000 to 30,000 and evaluation of different regression models for the interpolation and extrapolation of critical values: Revista Mexicana de Ciencias Geológicas, 25(3), 369–381. [ Links ]
Verma, S.P., Quiroz–Ruiz, A., Díaz–González, L., 2008, Critical values for 33 discordancy test variants for outliers in normal samples up to sizes 1000, and applications in quality control in Earth Sciences: Revista Mexicana de Ciencias Geológicas, 25(1), 82–96. [ Links ]
Vocke, R.D.Jr., 1999, Atomic weights of the elements 1997: Pure and Applied Chemistry, 71, 1593–1607. [ Links ]














