Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Sociedad Química de México
versión impresa ISSN 0583-7693
Rev. Soc. Quím. Méx vol.48 no.4 Ciudad de México oct./dic. 2004
Investigación
Intramolecular Radical Addition to the 2-Pyridone Nucleus
Yazmín M. Osornio,a* Luis D. Miranda,a and Joseph. M. Muchowskib*
a Instituto de Química, Universidad Nacional Autónoma de México, Circuito Exterior, Ciudad Universitaria, Coyoacán 04510, México, D.F.
b Chemistry, Roche Palo Alto, 3431 Hillview Avenue, Palo Alto, CA 94304-1320, USA. Tel: 55 56 22 44 08. E-mail: osornio_yaz@correo.unam.mx
Recibido el 27 de septiembre del 2004.
Aceptado el 17 de noviembre del 2004.
Abstract
The radical species derived from the N-ω-haloalkylpyridones 4b, 6c and the xanthates 7a, 7b undergo oxidative cyclization to the bicyclic 2-pyridone derivatives 8a-c irrespective of whether they are generated under reductive (Bu3SnH/AIBN) or oxidative [Fe(II)/H2O2/DMSO or DLP] conditions.
Key words: 2-pyridone, radical cyclization, Fenton, xanthates, dilauroyl peroxide.
Resumen
Los radicales alquilo generados a partir de los correspondiente N-ω-haloalquilpiridonas así como de sus correspondientes xantatos, sufren ciclación oxidativa y generan las correspondientes piridonas fusionadas. Los mismos productos fueron observados tanto en condiciones reductoras (Bu3SnH/AIBN) como en condiciones oxidantes [Fe(II)/H2O2/DMSO o DLP].
Palabras clave: 2-piridona, ciclación radical, Fenton, xantatos, peróxido de dilauroilo.
This paper is dedicated with affection to the memory of Dr. Raymundo Cruz Almanza.
Introduction
Enormous progress both in the mechanistic understanding and in the synthetic utility of free radical reactions has been achieved during the past two decades. One of the notable synthetic processes, which has been developed, is the cyclization of carbon-centered radicals to heteroaromatic systems followed by an in situ oxidative restoration of the aromaticity (oxidative radical cyclization) [1-4]. Several methods have been devised to effect such radical cyclizations, with n-Bu3SnH-mediated reactions being the most explored [2]. The highly toxic nature of the tin reagent, and the difficulties associated with the removal of the tin containing reaction products, have led synthetic organic chemists to find alternative methods to accomplish such reactions. In this context, we have recently utilized efficient "tin-free" methods in which organic peroxides serve as both the radical initiators and the oxidants, when alkyl iodides or the corresponding xanthates were used as the radical precursors [5]. Indeed, these processes were successfully applied to oxidative radical cyclizations on benzenoid, 4-quinolone, 1-isoquinolone, pyrrole and indole systems [5]. The prominent role played by pyridones and related compounds as privileged structures in many natural products and bioactive compounds, led us to consider the extension of our studies to the 2-pyridone nucleus. This process, if successful, would constitute a facile entry into the very important indolizidine and quinolizidine alkaloids (e. g., lupinine 1 and monomorine 2) [6]. In this paper we describe some of our results concerning the radical cyclization to the 2-pyridone nucleus using both reductive (n-Bu3SnH) and Fenton-type [3] and dilauroyl peroxide (DLP) based oxidative conditions.

Results and Discussion
The iodides 6 and xanthates 7 required for the radical cyclizations were synthesized in two steps by N-alkylation [7] of the 2-pyridones 3 with the appropriate α, ω-dibromoalkane followed by either, halogen exchange with an excess of sodium iodide or, nucleophilic substitution with potassium ethyl xanthate (Scheme 1). As was observed in the 1-isoquinolone series [7], the O-alkylated isomers 5a and 5c were formed as minor byproducts during the preparation of 4a and 4c. This was of little consequence since these compounds were transformed into the iodides 6a and 6c under the equilibrating conditions of the halogen exchange reaction. Curiously, the bromide 4b was destroyed under these conditions, but this too was of little importance since it was sufficiently reactive both to give the xanthate 7a and to participate in radical cyclization reactions.
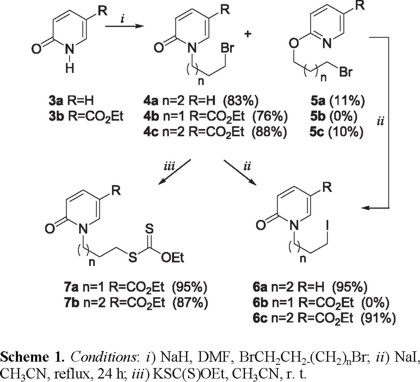
The radical reactions of 4b, 6a and 6c were first examined using n-Bu3SnH/AIBN in benzene at reflux temperature. Both 4b and 6c gave the products of oxidative radical cyclization 8b and 8c in 45 % and 11 % yields, respectively, accompanied by substantial amounts of the reductive dehalogenation products 9b and 9c (Table 1, Scheme 2). In contrast, the iodide 6a gave a complex mixture from which only the reductively dehalogenated compound 9a could be isolated. According with these results we suggest that the presence of the strongly electron withdrawing CO2Et (σ = 0.37) group at C-5 (e.g. 4b and 6c) in the pyridone ring system decrease the SOMO-LUMO energy difference at C-6 and favor radical attack at this site [2m]. The generation of 8b and 8c under these formally reducing conditions can no longer be considered unusual, although the nature of the oxidant can sometimes be obscure [2k]. For the examples described herein however, it is likely that AIBN is the oxidant, given that stoichiometric amounts of this radical initiator were required to achieve total consumption of the starting materials [2k].
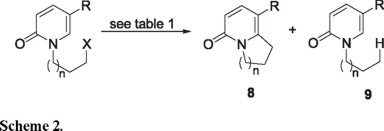
We next turned our attention to the use of the Fenton-type conditions [Fe(II)/H2O2/DMSO], which have given excellent results in pyrrole, and indole systems [3]. Compounds 4b and 6c did give rise to the expected products 8b and 8c, but in exceedingly poor yields. The iodide 6a gave a complex mixture of substances which did not contain 8a. These disappointing results encouraged us to examine the xanthates 7a and 7b as radical precursors with DLP as the initiator, under conditions developed by Zard, et. al [4]. Using stoichiometric quantities of DLP in 1,2-dichlorethane at reflux temperature, both 7a and 7b produced the expected bicyclic 2-pyridone derivatives 8b and 8c. Whereas 8b was obtained in a creditable 65 % yield, 8c was produced with considerably less efficiency (28 %), and was admixed with a significant quantity of the dithiocarbonate 11 (Scheme 3). The formation of 11 implies that the rate of attack of the radical 10 on the xanthate 7b (Scheme 3, path β) is competitive with its cyclization to 8b (Scheme 3, path α).

Although the radical cyclizations described herein were generally of modest efficiency, nevertheless they are the first reported examples of the intramolecular addition of alkyl radicals to the 2-pyridone system. It is noteworthy that such cyclizations have previously been reported to fail under typical n-Bu3SnH/AIBN mediated conditions [8].
Conclusions
The first examples of the intramolecular oxidative addition of alkyl radicals to the 2-pyridone system are described.
Experimental Section
The starting materials were purchased from Aldrich Chemical Co. and were used without further purification. Solvents were distilled before using them. Silica gel (230-400 mesh) was purchased from Merck. Silica plates of 0.20 mm thickness were used for thin layer chromatography. Melting points were determined with a Fisher-Johns melting point apparatus and they are uncorrected. 1H and 13C NMR spectra were recorded using a Varian Gemini 200, the chemical shifts (δ) are given in ppm relative to TMS as internal standard (0.00). For analytical purposes the mass spectra were recorded on a JEOL JMS-5X 10217 in the EI mode, 70 eV, 200 °C via direct inlet probe. IR spectra were recorded on a Nicolet Magna 55-X FT instrument.
Synthesis of the Bromo Compounds 4a-4c. To a suspension of NaH (60% in mineral oil, 1.2 equiv), in anhydrous DMF (1 mL/equiv substrate) and DME (4 mL/equiv substrate) was added a solution of the N-unsubstituted pyridone (1 equiv) in DME at 0 ºC. After 10 min, the reaction mixture was treated with LiBr (2 equiv) and stirred for 15 min, then α,ω-dibromoalkane in DME was added dropwise. The reaction mixture was stirred at room temperature for 1-6 h and then quenched with ice water. The mixture was extracted with ethyl acetate, the combined organic layer was washed successively with water and saturated sodium chloride solution, dried over sodium sulfate, and concentrated in vacuo. The residue was purified by column chromatography on silica gel using hexane-ethyl acetate mixtures to elute the product.
1-(4-Bromobutyl)-1H-pyridin-2-one (4a). Eluted with hexane-ethyl acetate (7:3) in 83% yield as an oil; IR (CHCl3) νmax: 2945, 2866, 1657, 1588, 1538 cm-1; 1H NMR (CDCl3, 300 MHz) δ 1.88-1.95 (4H, m), 3.41-3.47 (2H, m), 3.98 (2H, t, J = 7.0 Hz), 6.20 (1H, t, J =6.7 Hz), 6.57 (1H, d, J=8.8 Hz), 7.28-7.38(2H, m); 13C NMR (CDCl3, 75 MHz) δ 27.8, 29.4, 32.9, 48.6, 106.2, 120.8, 137.3, 139.4, 162.5; MS (EI) m/z (rel. intensity) 229 (M+, 25), 231 (24), 150 (100).
Ethyl 1-(3-Bromopropyl)-1H-pyridin-2-one-5-carboxylate (4b). Eluted with hexane-ethyl acetate (1:1) in 76% yield as a white solid mp 75-76 ºC; IR (KBr) νmax: 2980, 2870, 1714, 1665, 1609, 1543 cm-1; 1H NMR (CDCl3, 300 MHz) δ 1.37 (3H, t, J = 7.2 Hz), 2.36 (2H, quintet, J = 6.8 Hz), 3.43 (2H, t, J = 6.8 Hz), 4.15 (2H, t, J = 6.8 Hz), 4.33 (2H, q, J = 7.2 Hz), 6.54 (1H, d, J = 10.0 Hz), 7.87 (1H, dd, J = 2.2, 10.0 Hz), 8.23 (1H, d, J = 2.2 Hz); 13C NMR (CDCl3, 75 MHz) δ 14.1, 29.6, 30.9, 49.1, 60.9, 110.0, 119.6, 138.6, 142.8, 162.2, 163.8; MS (EI) m/z (rel. intensity) 287 (M+, 10), 289 (9), 208 (100).
Ethyl 1-(4-Bromobutyl)-1H-pyridin-2-one-5-carboxylate (4c). Eluted with hexane-ethyl acetate (8:2) in 76% yield as a white solid mp 58-59ºC; IR (KBr) νmax: 2959, 2875, 1712, 1656, 1608, 1540cm-1; 1H NMR (CDCl3, 300 MHz) δ 1.37 (3H, t, J = 7.2 Hz), 1.91-1.96 (4H, m), 3.45 (2H, t, J = 6.8 Hz), 4.03 (2H, t, J = 6.8 Hz), 4.33 (2H, q, J = 7.2 Hz), 6.58 (1H, d, J = 10 Hz), 7.87 (1H, dd, J = 10.0, 2.2 Hz), 8.18 (1H, d, J = 2.2 Hz); 13C NMR (CDCl3, 75 MHz) δ 14.3, 27.9, 29.5, 32.5, 49.4, 61.1, 110.3, 119.8, 138.5, 142.5, 162.4, 164.2; MS (EI) m/z (rel. intensity) 301 (M+, 15), 303 (14), 222 (100).
Synthesis of the Iodides 6a, 6c. A solution of the bromide (1 equiv) in acetonitrile (30 mL/g bromide) containing sodium iodide (3-4 equiv) was heated at reflux temperature for 6-24 h. The solution was poured into water and extracted with dichloromethane. The extract was washed with saturated aqueous sodium sulfite solution and water, and then it was dried over sodium sulfate. The solvent was removed in vacuo, and the residue was purified by column chromatography on silica gel using hexane-ethyl acetate mixtures to elute the product.
1-(4-Iodobutyl)-1H-pyridin-2-one (6a). Eluted with hexane-ethyl acetate (7:3) in 95% yield as an oil; IR (CHCl3) νmax: 2943, 2862, 1658, 1586, 1537cm-1; 1H NMR (CDCl3, 300 MHz) δ 1.84-1.91 (4H, m), 3.18-3.25 (2H, m), 3.96 (2H, t, J = 7.0 Hz), 6.18 (1H, t, J = 6.7 Hz), 6.56 (1H, d, J = 8.8 Hz), 7.23-7.36 (m, 2H); 13C NMR (CDCl3, 75 MHz) δ 5.8, 30.1, 30.4, 48.4, 106.1, 120.8, 137.1, 139.4, 162.5; MS (EI) m/z (rel. intensity) 277 (M+, 25), 150 (100).
Ethyl 1-(4-Iodobutyl)-1H-pyridin-2-one-5-carboxylate (6c). Eluted with hexane-ethyl acetate (7:3) in 80% yield as a pale yellow solid mp 65-67ºC; IR (KBr) νmax: 2959, 1712, 1656, 1607, 1539 cm-1; 1H NMR (CDCl3, 300 MHz) δ 1.37 (3H, t, J = 7.2 Hz), 1.86-1.91 (4H, m), 3.22 (2H, t, J = 6.8 Hz), 4.00 (2H, t, J = 6.8 Hz), 4.34 (2H, q, J = 7.2 Hz), 6.53 (1H, d, J = 10 Hz), 7.85 (1H, dd, J = 10.0, 2.2 Hz), 8.14 (1H, d, J = 2.2 Hz); 13C NMR (CDCl3, 75 MHz) δ 5.1, 14.3, 30.1, 30.2, 49.2, 61.0, 110.2, 119.8, 138.5, 142.4, 162.4, 164.2; MS (EI) m/z (rel. intensity) 349 (M+, 15), 222 (100); HRMS (FAB+): calcd for C12H17INO3: 350.0253, found: 350.0247.
Synthesis of the Xanthates 7a and 7b. A solution of the bromide (1 equiv) in acetonitrile (30 mL/g bromide) containing O-ethylxanthic acid, potassium salt (1.2 equiv) was stirred at room temperature for 2 h. The solution was poured into water and extract with dichlorometane. The extract was washed with water, and then it was dried over sodium sulfate. The solvent was removed in vacuo, and the residue was purified by column chromatography on silica gel using hexane-ethyl acetate mixtures to elute the product.
Dithiocarbonic acid O-ethyl ester S-[3-(3-carboethoxy-2-oxo-1H-pyridin-1-yl)-propyl]ester (7a). Eluted with hexane-ethyl acetate (8:2) in 95% yield as a white solid mp 49-50 ºC; IR (KBr) νmax: 2937, 2871, 1715, 1664, 1612, 1543 cm-1; 1H NMR (CDCl3, 300 MHz) δ 1.37 (3H, t, J = 7.2 Hz), 1.42 (3H, t, J = 7.2 Hz), 2.2 (2H, quintet, J = 7.0 Hz), 3.17 (2H, t, J = 7.0 Hz), 4.07 (2H, t, J = 7.0 Hz), 4.34 (2H, q, J = 7.2 Hz), 4.64 (2H, q, J = 7.2 Hz), 6.54 (1H, d, J = 10 Hz), 7.85 (1H, dd, J = 10.0, 2.2 Hz), 8.14 (1H, d, J = 2.2 Hz); 13C NMR (CDCl3, 75 MHz) δ 13.7, 14.3, 28.0, 32.4, 49.3, 61.0, 70.1, 110.1, 119.8, 138.6, 142.6, 162.3, 164.0, 213.9; MS (EI) m/z (rel. intensity) 329 (M+, 55), 208 (100); HRMS (FAB+): calcd for C14H20O4NS2: 330.0834, found: 330.0834.
Dithiocarbonic acid O-ethyl ester S-[4-(3-carboethoxy-2-oxo-1H-pyridin-1-yl)-butyl]ester (7b). Eluted with hexane-ethyl acetate (7:3) in 87% yield as a white solid mp 65-66 ºC; IR (KBr) νmax: 2940, 1714, 1666, 1610, 1543, 1445, 1292 cm-1; 1H NMR (CDCl3, 300 MHz) δ 1.36 (3H, t, J = 7.2 Hz), 1.41 (3H, t, J = 7.2 Hz), 1.72-1.95 (m, 4H), 3.17 (2H, t, J = 6.9 Hz), 4.02 (2H, t, J = 6.9 Hz), 4.31 (2H, q, J = 7.2 Hz), 4.64 (2H, q, J = 7.2 Hz), 6.54 (1H, d, J = 10 Hz), 7.84 (1H, dd, J = 10.0, 2.2 Hz), 8.15 (1H, d, J = 2.2 Hz); 13C NMR (CDCl3, 75 MHz) δ 13.7, 14.2, 25.5, 28.3, 35.0, 49.8, 61.0, 69.9, 110.1, 119.6, 138.5, 142.5, 162.3, 164.1, 214.5; MS (EI) m/z (rel. intensity) 343 (M+, 5), 222 (100); HRMS (FAB+): calcd for C15H22O4NS2: 344.0990, found: 344.0995.
General Procedure for Radical Cyclization Using Dilauroyl Peroxide. To a degassed solution of the corresponding xanthate derivative (1.0 equiv) in refluxing dichloroethane (7 mL/mmol) was added dilauroyl peroxide (1.2 equiv) portionwise (0.3 equiv/1.5 h). The reaction was carried out under an atmosphere of N2 during 7.5 h. Then, the solvent was removed in vacuo, and the residue was purified by column chromatography on silica gel using hexane-ethyl acetate mixtures to elute the product.
Ethyl 4-oxo-6,7,8,9-tetrahydro-4H-quinolizine-1-carboxylate (8b). Eluted with hexane-ethyl acetate (7:3) in 65% yield as a white solid mp 102-104 ºC; IR (KBr) νmax: 1686, 1643 cm-1; 1H NMR (CDCl3, 300 MHz) δ 1.34 (3H, t, J = 7.1 Hz), 2.22 (2H, quintet, J = 7.6 Hz), 3.54 (2H, t, J = 7.9 Hz), 4.17 (2H, t, J = 7.4 Hz), 4.29 (2H, q, J = 7.1 Hz), 6.48 (1H, d, J = 9.5 Hz), 7.92 (1H, d, J = 9.5 Hz); 13C NMR (CDCl3, 75 MHz) δ 20.5, 33.8, 49.4, 60.8, 106.6, 116.4, 140.6, 157.3, 162.2, 164.6; MS (EI) m/z (rel. intensity) 207 (M+, 100).
Ethyl 4-oxo-6,7,8,9-tetrahydro-4H-quinolizine-1-carboxylate (8c). Eluted with hexane-ethyl acetate (6:4) in 28% yield as a white solid mp 54-56ºC; IR (KBr) νmax: 1701, 1650 cm-1; 1H NMR (CDCl3, 300 MHz) δ 1.36 (3H, t, J = 7.2 Hz), 1.41 (3H, t, J = 7.2 Hz), 1.77-2.01 (m, 4H), 3.38 (2H, t, J = 6.4 Hz), 4.06 (2H, t, J = 6.2 Hz), 4.29 (2H, q, J = 7.2 Hz), 6.46 (1H, d, J = 9.5 Hz), 7.92 (1H, d, J = 9.5 Hz); 13C NMR (CDCl3, 75 MHz) δ 14.2, 18.1, 21.3, 26.7, 42.3, 60.5, 107.7, 115.2, 139.6, 155.5, 162.8, 165.2; MS (EI) m/z (rel. intensity) 221 (M+, 100); HRMS (FAB+): calcd for C12H16NO3: 222.1130, found: 222.1123.
Acknowledgments
We thank DGAPA-UNAM (PAPIIT-IN228103) for generous financial support. Also we thank Rocío Patiño, Elizabeth Huerta, Angeles Peña, Javier Pérez, Luis Velasco and Nieves Zavala, for their technical support.
References
1. For reviews in this field see: (a) Studer, A. in Radicals in Organic Synthesis, Renaud, P and Sibi, M. Ed. Wiley VCH, Weinhem, 2001; Vol. 2, p. 62-76, [ Links ] (b) Bowman, W. R.; Bridge, C. F.; Brookes, P. J. Chem. Soc., Perkin Trans.1. 2000, 1-14, [ Links ] (c) Bowman, W. R.; Fletcher, A. J.; Potts, G. B. S. J. Chem Soc., Perkin Trans. 1, 2002, 2747-2762. [ Links ]
2. Recent publications: (a) Aldabbagh, F.; Bowman, W. R.; Mann, E.; Slawin, A. M. Z. Tetrahedron. 1999, 55, 8111-8128, [ Links ] (b) Nadin, A.; Harrison, T. Tetrahedron Lett. 1999, 4073-4076, [ Links ] (c) Marco-Contelles, J.; Rodríguez-Fernández, M. Tetrahedron Lett. 2000, 41, 381-384, [ Links ] (d) Bowmann, W. R.; Mann, E.; Parr, J. J. Chem. Soc., Perkin Trans. 1. 2000, 2991-2999, [ Links ] (e) Miranda, L. D.; Cruz-Almanza, R.; Pavón, M.; Romero, Y.; Muchowski, J. M. Tetrahedron Lett. 2000, 41, 10181-10184, [ Links ] (f) McCarroll, A. J., Walton, J. C. J. Chem Soc., Perkin Trans. 1. 2001, 3215-3229, [ Links ] (g) Bennasar, M.-Ll., Roca, T., Griera, R., Bosch J. J. Org. Chem. 2001, 66, 7547-7551, [ Links ] (h) Bennasar, M.-Ll., Roca, T., Griera, R., Bosch J. J. Org. Chem. 2002, 67, 6268-6271, [ Links ] (i) Allin, S. M.; Barton, W. R. S.; Bowman, W. R.; McInally, T. [ Links ] Tetrahedron Lett. 2002, 43, 4191-4193, (j) Rheault, T. R.; Sibi, M. P. Synthesis, 2003, 803-819, [ Links ] (k) Beckwith, A. L. J.; Bowry, V. W.; Bowman, W. R; Mann, E.; Parr, J.; Storey, J. M. D. Angew. Chem., Int. Ed. 2004, 43, 95-98, [ Links ] (l) Bennasar, M.-Ll.; Roca T.; Ferrando, F. Tetrahedron Lett. 2004, 45, 5605-5609, [ Links ] (m) Osornio, Y. M.; Miranda, L. D.; Cruz-Almanza, R.; Muchowski, J. M. Tetrahedron Lett. 2004, 45, 2855-2858. [ Links ] For cyclizations under related formal oxidative conditions see: (n) Mohan, R.; Kates, S. A.; Dombroski, M. A.; Snider, B. B. Tetrahedron Lett. 1987, 28, 845-848, [ Links ] (o) Snider, B. B.; Buckman, B. O. Tetrahedron. 1989, 45, 6969-6978 and references cited therein, [ Links ] (p) Aidhen, I. S.; Narasimhan, N. S. Tetrahedron Lett. 1989, 30, 5323-5324, [ Links ] (q) Artis, D. R.; Cho. I-S and Muchoswki. J. M. Can. J. Chem. 1992, 70, 1838-1842, [ Links ] and references cited therein.
3. For cyclizations under Fenton-type oxidative conditions: (a) Artis, D. R.; Cho. I-S.; Figueroa, S. J.; Muchoswki, J. M. J. Org. Chem. 1994, 59, 2456-2466, [ Links ] (b) Miranda, L. D.; Cruz-Almanza, R.; Pavón, M. ARKIVOC. 2002, 15-22. [ Links ]
4. For cyclizations using xanthate-based chemistry see: (a) Gagosz, F.; Zard, S. Z. Organic Lett. 2002, 4, 4345-4348, [ Links ] (b) Axon, J.; Boiteau, L.; Boivin, J.; Forbes, J. E. and Zard, S. Z. Tetrahedron Lett. 1994, 35, 1719-1722, [ Links ] (c) Liard, A.; Quiclet-Sire, B.; Saicic, R. N.; Zard, S. Z. Tetrahedron Lett. 1997, 38, 1759-1762, [ Links ] (d) Cholleton, N.; Zard, S. Z. Tetrahedron Lett. 1998, 39, 7295-7298, [ Links ] (e) Ly, T. M.; Quiclet-Sire, B.; Sortais, B.; Zard, S. Z. Tetrahedron Lett. 1999, 40, 2533-2536. [ Links ]
5. Menes-Arzate, M.; Martínez, R.; Cruz-Almanza, R.; Osornio, Y. M.; Muchowski, J. M.; Miranda, L. D. J. Org. Chem. 2004, 69, 4001-4004. [ Links ]
6. For a review on Indolizidine alkaloids see: Takahata, H.; Mamose, T. in The Alkaloids, Ed. Cordell, G. A., vol. 44, pp. 189-256. [ Links ] For a review on Quinolizidine alkaloids see: Johne, S. in The Alkaloids, Ed. Cordell, G. A., vol. 47, pp. 1-114. [ Links ]
7. Hiu, L.; Sung-Bo, K.; Josien, H.; Curran, D. P. Tetrahedron Lett. 1995, 36, 8917-8920, [ Links ] (b) Rico, I.; Halvorsen, K.; Dubrule, C.; Lattes, A. J. Org. Chem. 1994, 59, 415-420. [ Links ]
8. Nadin, A.; Harrison, T. Tetrahedron Lett. 1999, 40, 4073-4076. [ Links ]














