Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Sociedad Química de México
versión impresa ISSN 0583-7693
Rev. Soc. Quím. Méx vol.48 no.4 Ciudad de México oct./dic. 2004
Investigación
Synthesis of Potential Anti-inflammatory Compounds, Selective Inhibitors of Cyclooxygenase-2 (COX-2)
Angel Guzmán,*1 Eduardo Díaz,1 Blanca E. Trejo1 and Francisco J. López-Muñoz2
1 Instituto de Química, Universidad Nacional Autónoma de México, Circuito Exterior, Ciudad Universitaria, Coyoacán, 04510. México, D. F. E-mail: angelgs@servidor.unam.mx
2 Laboratorio Dolor y Analgesia, Departamento de Farmacobiología, Centro de Investigación y Estudios Avanzados del Instituto Politécnico Nacional.
Recibido el 19 de mayo del 2004.
Aceptado el 30 de agosto del 2004.
Abstract
Seven compounds were synthesized as potential anti-inflammatory drugs, selective inhibitors of the enzyme cyclooxygenase-2 (COX-2). The structural design was based on the knowledge of the active site of the enzyme. The synthesized compounds present a combined naproxen and celecoxib structures.
Key words: Cyclooxygenase, anti-inflammatory drugs, naproxen, celecoxib, synthesis.
Resumen
Se sintetizaron siete compuestos como drogas potencialmente anti-inflamatorias, inhibidores selectivos de la enzima ciclooxigenasa-2 (COX-2). El diseño estructural se basó en el conocimiento del sitio activo de la enzima. Los compuestos sintetizados presentan estructuras combinadas de naproxen y celecoxib.
Palabras clave: ciclooxigenasa, drogas antiinflamatorias, naproxen, celecoxib, síntesis.
This paper is dedicated to the memory of Dr. Raymundo Cruz Almanza
The non-steroidal anti-inflammatory drugs (NSAID's), developed in the 60's, were well accepted as a treatment for rheumatoid arthritis, osteoarthritis and pain. However, these drugs tend to cause significant side effects, which include gastric and intestinal toxicity as well as a decrease in the renal function [1].
The demonstration that inhibition of prostaglandin synthesis, via a cyclooxygenase (COX) enzyme, is the mechanism of action of non-steroidal anti-inflammatory drugs, was the central issue in understanding both the therapeutic and toxic effects of these substances. Cyclooxygenase is the enzyme that catalyzes the biological oxidation of arachidonic acid to prostaglandins, which are biosynthesized in practically all tissues of the human body, eliciting a variety of pharmacological effects, some of them beneficial, as support of renal and platelet functions, gastrointestinal protection, and other non-beneficial as pain, fever and other symptoms associated with the inflammatory response.
It was long held that cyclooxygenase was a single enzyme present in most cells. More recently, two forms of cyclooxygenase have been found: COX-1 and COX-2. COX-1 is a constitutive form found in the intestine, stomach, kidneys and platelets, while COX-2 is an inducible form, expressed during inflammation [2]. Existing nonsteroidal antiinflamatory drugs inhibit both forms of the enzyme, thus blocking both the positive and negative effects of prostaglandins.
Based on this discovery, it was proposed that a selective inhibitor of COX-2 would be an attractive approach for the treatment of inflammatory conditions, discarding side effects as gastric ulcers and renal toxicity [2]. Recently, two compounds with good selectivity as inhibitors of COX-2, came out to the market: celecoxib (1) and rofecoxib (2) (Scheme 1).
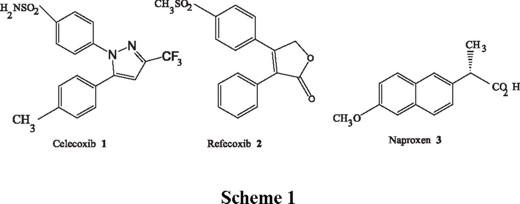
Celecoxib showed excellent selectivity for COX-2 over COX-1 in vitro ( IC 50: 0.04 vs. 15.0 µM, respectively). In a number of in vivo models, celecoxib demonstrated potent anti-inflammatory activity after oral dosing, decreasing acute inflammation in the carrageenan-induced edema assay (ED50: 7.1 mg/Kg) [1]. These results, although similar to those obtained with standard NSAID's, demonstrated that celecoxib produced neither acute nor chronic gastrointestinal toxicity in rats at the respective doses of up to 600 mg/Kg/day during 10 days. Celecoxib had a good bioavailability, as well as an excellent safety profile in these preclinical models.
On the other hand, Rofecoxib showed to be highly specific for COX-2 in a randomized, double blind trial, in which it was orally administered to volunteers, 25 mg once a day, vs. naproxen 500 mg. The results showed that while naproxen inhibited gastric prostaglandin synthesis by 70%, rofecoxib had no effects on these parameters [3]. In other studies, patients with osteoarthritis showed a lower incidence of upper gastrointestinal ulcers after treatment with rofecoxib at 12.5, 25 or 50 mg once a day, compared to patients treated with ibuprofen 800 mg daily, during 6 weeks.
Considering the recent discovery of COX-2 as well as the inhibitors of this enzyme, we established the hypothesis that other chemical structures should have similar pharmacological properties as celecoxib or rofecoxib, but with a better profile, less side reactions and more selectivity; the discovery of these molecules will be very important for handling and treatment of rheumatic diseases such as arthritis, osteoarthritis, inflammation and pain.
The objective of this work was to prepare a series of compounds in which we incorporated part of the structure of naproxen and part of the rofecoxib or celecoxib, and their biological evaluation as anti-inflammatory drugs.
COX-1 and COX-2 are two very similar proteins, consisting of a long narrow channel with a hairpin bend at the end. For COX-1, arachidonic acid is sucked in on the channel; in the inner of the channel a residue of serine 530 can be found, placed so to control the access of arachidonic acid, then there is as well, a residue of arginine 120, used to bind the carboxylic acid group of the fatty acid substrate. When arachidonic acid is bound, a residue of tyrosine 385 thought initiate the COX oxidation reaction, resulting in the five-carbon ring that characterizes prostaglandins [4]. The nonsteroidal anti-inflammatory drugs, most of which are also carboxylic acids are also considered to bind to this arginine 120.
Several lines of experimental evidence have suggested that the substrate binding site for fatty acids to be oxidized at the active site in COX-2 is larger than that in COX-1 [5,6]. For COX-2, the reactive site also consists of a long narrow channel [7], a serine 530 lies halfway along the tunnel and controls the access of arachidonic acid. The arginine 120 is also present, but this is not the site of binding for the selective inhibitors of COX-2 since these compounds do not have a carboxylic group. It is the nearby single aminoacid difference that is critical for the selectivity of many drugs; at position 523 an isoleucine molecule in COX-1 and a valine in COX-2 are located. The smaller valine molecule in COX-2 leaves a gap in the wall of the channel, giving access to a side pocket, which is thought to be the site of binding for the selective drugs. The bulkier isoleucine at 523 in COX-1 is large enough to block access to the side pocket. Targeted single aminoacid substitution of valine for isoleucine is sufficient to turn COX-1 into an enzyme that can be inhibited by COX-2 selective agents.
Considering the before mentioned, a COX-2 inhibitor should have a structure that enter the channel formed by the enzyme and bind within the COX-2 side pocket, often via sulfonyl, sulfone, or sulfonamide groups to achieve selectivity.
Naproxen 3 is a COX-1 inhibitor; this substance has been an excellent anti-inflammatory drug in clinical use for more than 20 years. The chemical structure of this compound corresponds to the first generation of NSAID's; a flat ring, a naphthalene in this case and a 2-propionic acid fragment at C-2. Based on the good pharmacological properties of this compound, we established the hypothesis that combining the bulky naphthalene ring of naproxen, which we assume would enter easily into the active channel of COX-1 or COX-2, and structural fragments of celecoxib or rofecoxib, which would bind within the COX-2 side pocket, we would be able to obtain potent and selective inhibitors of COX-2, which would be expected to have good anti-inflammatory properties.
This paper describes the synthesis of compounds 6 and 6c to 6h (scheme 2) which were designed taken in consideration the structure of the active site of the enzyme and our hypothesis. Compound 6 was prepared starting with 2-methoxynaphthalene 4, which was transformed in the acetyl derivative 5 by a Friedel-Crafts acylation [8]. Condensation of 5 with isopropyl trifluoroacetate in presence of sodium methoxide gave diketone 5a in 55% yield. Reaction of 5a with phenylhydrazine hydrochloride provided pyrazol 6 in 91% yield. The orientation of the phenyl ring was assigned on the base of other examples [9], and was confirmed by X ray diffraction (vide infra). Cleavage of the ether group with hydrobromic acid in acetic acid solution afforded phenol 6a in 97% yield. Phenol 6a was transformed into the thiol 6d by rearrangement of O-aryl dialkylthiocarbamate to S-aryl dialkylthiocarbamate, followed by basic hydrolysis [10]. Thus, compound 6a was reacted with dimethylthiocarbamoyl chloride to give the derivative 6b in 85% yield. Heating 6b at 270-280°C for 1.5 h followed by purification, gave 6c in 73% yield, which was hydrolyzed with sodium hydroxide in methanol solution to afford the thiol 6d in 90% yield. Thiol 6d was transformed into the methyl thioether [11] 6e by treatment with methyl iodide and potassium carbonate in DMF solution (77%), and this into the sulfoxide 6f [12] and the sulfone [13] 6g by reaction with sodium periodate (93%) and m-chloroperbenzoic acid (89%) respectively. In order to confirm the position of the phenyl substituent around the pyrazol ring, sulfone 6g was submitted to X ray diffraction, this experiment was in agreement with the preliminary structural assignment (fig.1).
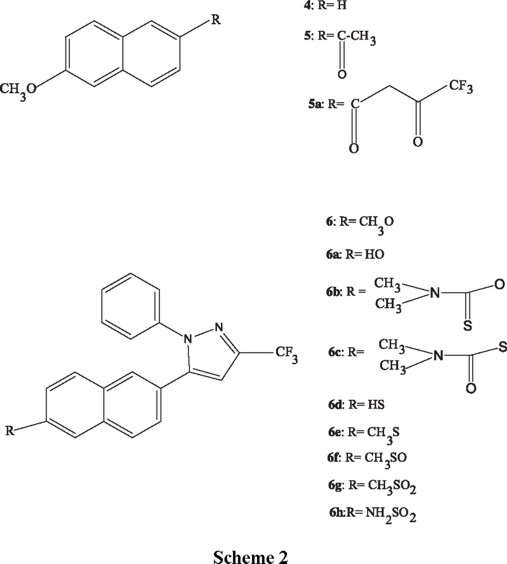
Trying to synthesize sulfonamide 6h, the S-aryl dimethylthiocarbamate 6c was oxidized with hydrogen peroxide in formic acid solution [14], but despite of the attempts, the reaction was unsuccessful. After several experiments, we found that treating the thiol 6d with chlorine and hydrogen peroxide, it is converted to the corresponding aryl sulfonyl chloride [15], which turns into sulfonamide 6h in 53% yield by reaction with aqueous ammonia .
The synthesized compounds were submitted to biological evaluation. They are being evaluated by the PIFIR method [16] and the results will be presented elsewhere.
Experimental section
All starting materials were commercially available research grade chemicals and were used without further purification. Silica gel 60 F254 was used for TLC and the spots were detected with UV. Flash column chromatography was carried out in silica gel 200-325 mesh. IR spectra were taken on Nicolet FX-SX and Nicolet 55-X spectrophotometers in CHCl3 solution, unless otherwise stated. The NMR spectra were obtained with a Varian Gemini 200 and Unity 300 spectrometers using chloroform as solvent and TMS as an internal reference. Mass spectra were taken in a Jeol JMS-AX505 HA instrument. Melting points were obtained on a Büchi 510 and are uncorrected.
4,4,4-Trifluoro-1-(6-methoxy-2-naphthalenyl)-1,3-butanodione (5a). To a solution of 6-methoxy-2-acetonaphtone 5, 28.7 g, 0.14 mol) in 130 mL of 1,2-dimetoxyethane was added 7.5 g (0.14 mol) of sodium methoxide and 37.22 g, (0.23 mol) of isopropyl trifluoroacetate. The solution was heated under reflux for 16 h and then 50 mL of water were added, the pH adjusted to 1.0 with aqueous HCl and extracted with methylene chloride, the combined extracts were washed with water, dried over anhydrous sodium sulfate. The methylene chloride was evaporated and the residue crystallized from methylene chloride-hexane to give 23.6 g (55% yield) of 5a, mp 85-87°. IR (KBr): 1620, 1483, 1276,1194 cm-1; 1H NMR (CDCl3, 300 MHz): δ 3.96 (s, 3H), 6.68 (s, 2H), 7.25 (m, 3H), 7.90 (m, 3H), 8.44 (s 1H); MS (EI), m/z 296 (M+)
1-Phenyl-3-trifluoromethyl-5-(6-methoxy-2-naphthalenyl)pyrazol (6). To a solution of 27 g (0.09 mol) of 5a in 200 mL of ethanol, was added 20 g (0.14 mol) of phenylhydrazine hydrochloride in 110 mL of water. The resulting solution was heated under reflux for 1 h and then poured into 200 mL of water. The product was isolated by methylene chloride extraction; the extracts were dried over sodium sulfate and evaporated under vacuo. The residue was purified by crystallization from methylene chloride-hexane to give 30.7 g (91%) of 6, mp 96-98°. IR (KBr): 1629, 1601, 1493, 1259, 1237 cm-1; 1H NMR (CDCl3, 300 MHz): δ 3.92 (s, 3H), 6.82 (s, 1H), 7.25 (m, 5H), 7.15 (m, 3H), 7.60 (m, 3H); MS (EI): m/z 368( M+).
1-Phenyl-3-trifluoromethyl-5-(6-hydroxy-2-naphthalenyl)pirazol (6a). To a solution of 6, 30.72 g, (0.83 mol) in 200 mL of acetic acid, were added 150 mL of 48% hydrobromic acid (0.89 mol). The solution was heated under reflux for 24 h and then diluted with 150 mL of water and extracted with methylene chloride; the extracts were dried over sodium sulfate and evaporated under vacuo. The residue was purified by column chromatography on 600 g of silica gel (hexane-ethyl acetate 9:1), yielding 28.7 g (97%) of 6a. IR (KBr): 3317, 1631, 1608, 1496, 1163 cm-1 ; 1H NMR (CDCl3, 300 MHz): δ 5.12 (s, 1H), 6.82 (s, 1H), 7.34 (m, 5H), 7.20 (m, 3H), 7.60 (m, 6H); MS (EI): m/z 354 (M+).
1-Phenyl-3-trifluoromethyl-5-(6-O-dimethylthionocarbamoyl-2-naphthalenyl)pyrazol (6b). To a solution of 28.67 g (0.08 mol) of 6a in 220 mL of DMF, 17.96 g (0.13 mol) of DABCO and 14.83 g, (0.12 mol) of dimethylthiocarbamoyl chloride was added. The mixture was heated at 30-35° for 30 min and then at 75° for 1 h. The reaction mixture was poured in 100 mL of cold water and extracted with methylene chloride. The extracts were washed with water, dried over sodium sulfate and evaporated under vacuo. The residue was crystallized from methanol to give 30.3 g (85%) of 6b, mp 118-120°; IR (KBr): 1539, 1487, 1236 cm-1; 1H NMR (CDCl3, 300 MHz): δ 3.40, 3.48 (2s, 6H), 6.85 (s, 1H), 7.35 (m, 5H), 7.20 (m, 3H), 7.70 (m, 3H); MS (EI): m/z 441 (M+).
1-Phenyl-3-trifluoromethyl-5-(6-S-dimethylthiocarbamoyl-2-naphthalenyl)-pyrazol (6c). Compound 6b, 30.3 g, (68 mmol) was heated in a round-bottomed flask to 270-280° for 1.5 hr. After cooling to rt the residue was chromatographed on 600 g of silica gel (Hexane-Ethyl acetate 98:2), giving 22 g (73%) of 6c, mp 83-84°. IR (KBr): 1662, 1362, 1237 cm-1; 1H NMR (CDCl3, 300 MHz): δ 3.06, 3.11 (2s, 6H), 6.86 (s, 1H), 7.25 (m, 5H), 7.20 (m, 3H),7.80 (m, 3H); MS (EI): m/z 441 (M+).
1-Phenyl-3-trifluoromethyl-5-(6-mercapto-2-naphthalenyl)pyrazol (6d). To a solution of 8 g (0.2 mol) of sodium hydroxide in 80 mL of methanol, 22 g (0.05 mol) of compound 6c was added. The mixture was heated under reflux for 10 min, allowed to cool, poured in 80 mL of water, acidified with 10% hydrochloric acid and extracted with methylene chloride. The extracts were washed with water, dried over sodium sulfate and evaporated. The residue was treated with charcoal to give 16.6 g (90%) of 6d, as a brown solid. IR (KBr): 2568, 1596, 1489, 1239 cm-1; 1H NMR (CDCl3, 300 MHz): δ 2.90 (s, 1H), 6.84 (s, 1H), 7.33 (m, 5H), 7.20, 760 (2m, 6H); MS (EI): m/z 370 (M+).
1-Phenyl-3-trifluoromethyl-5-(6-methylthio-2-naphthalenyl)pyrazol (6e). To a solution of 8.61 g (23.2 mmol) of 6d in 200 mL of DMF, 3.2 g (23.2 mmol) of potassium carbonate and 2.9 mL (6.612 g, 46.5 mmol) of methyl iodide were added. The mixture was stirred at rt for 30 min. The excess of DMF was evaporated under vacuo, the residue was diluted with 30 mL of water and extracted with methylene chloride. The extracts were washed with water, dried over sodium sulfate and evaporated. The residue was purified by column chromatography on 150 g of silica gel (hexane-ethyl acetate 98:2), yielding 6.9 g of compound 6e, (77% yield), mp 65-67°. IR (KBr): 1595, 1485, 1237, 1134 cm-1; 1H NMR (CDCl3, 300 MHz); δ 2.57 (s, 3H), 6.84 (s, 1H), 7.35 (m, 5H), 7.2 (m, 3H), 7.60 (m, 3H); MS (EI): m/z 384 (M+).
1-Phenyl-3-trifluoromethyl-5-(6-methylsulfinyl-2-naphthalenyl)pyrazol (6f). To a solution of 1 g, (2.6 mmol) of 6e in 20 mL of methanol, a solution of 725 mg (3.38 mmol) of sodium periodate in 1.5 mL of water was added . The reaction was stirred for 72 h at rt and then diluted with 10 mL of water, extracted with methylene chloride, the extracts were dried over sodium sulfate and evaporated. The residue was purified by preparative TLC (hexane-ethyl acetate 1:1), to obtain 970 mg (93%) of 6f as a white solid, mp 111-112°; IR (KBr): 1597, 1490, 1238, 1135, 1105 cm-1; 1H NMR (CDCl3, 300 MHz): δ 2.8 (s, 3H), 6.89 (s, 1H), 7.36 (m, 6H), 7.61 (m, 1H), 7.86 (m, 3H), 8.21 (d, 1H); MS (EI): m/z 400 (M+).
1-Phenyl-3-trifluoromethyl-5-(6-methylsulfonyl-2-naphthalenyl)-pyrazol (6g). To a solution of 3 g (7.2 mmol) of compound 6f in 50 mL of methylene chloride, 3.8 g (22 mmol) of m-chloroperbenzoic acid was added. The reaction was stirred for 24 h, diluted with 50 mL of methylene chloride and washed with saturated solution of sodium bicarbonate and water, dried with sodium sulfate and evaporated. The residue was purified by column chromatography on 60 g of silica gel (hexane-ethyl acetate 8:2), to give 2.9 g of 6g (89%), mp 138-139°; IR (KBr): 1597, 1497, 1301, 1131 cm-1; 1H NMR (CDCl3, 300 MHz) δ 3.12 (s, 3H), 6.92 (s, 1H), 7.26 (m, 6H), 7.35 (m, 2H), 7.93, (m, 3H) 8.5 (s, 1H); MS (EI): m/z 416 (M+).
6-[5-(1-phenyl-3-trifluoromethyl)-pyrazolyl)]-naphthalen-2-sulfonamide( 6h). A solution of 740 mg of compound 6d in 20 mL of methylene chloride was cooled to 0° and then 0.2 mL (3 mmol) of 50% hydrogen peroxide was added and 568 mg (8 mmol) of chlorine was bubbled. The reaction was stirred for 10 min, the phases were separated and the organic phase was poured in 14 mL of 28% ammonium hydroxide. The mixture was stirred for 10 min a 0°; the phases separated and the organic phase washed with water, dried over sodium sulfate and evaporated. The residue was purified by column chromatography in 15 g of silica gel (hexane-ethyl acetate 9:1), to give 439 mg of compound 6h (53% yield) as a white solid, mp 158-160°; IR (KBr): 1597, 1491, 1335, 1238, 1158 cm-1; 1H NMR (CDCl3, 300 MHz): δ 4.99 (s, 2H), 6.90 (s, 1H), 7.35 (m, 5H), 7.91 (m, 4H), 8.45 (s, 1H); MS (EI): m/z 417 (M+).
References
1. Graul, A.; Martel, A.M. Drugs of the future 1997, 22, 711-714. [ Links ]
2. Battistine, B.; Botting, R. Bankhle, Y.S.; Drugs News Perspect. 1994, 7, 501-512. [ Links ]
3. Cryer, B. Drugs of the future 1999, 24, 1374-1378. [ Links ]
4. Bakhle, Y. S. Drugs of Today 1999, 35, 237-250. [ Links ]
5. Loung, C.; Miller, A.; Barnett, J.; Chow, J. Ramesha, C.; Browne, M.F. Nat Struc. Biol. 1996, 3, 927-933. [ Links ]
6. Kurumball, R.G.; Stevens, A. M.; Gierse, J. K. Nature 1996, 384, 644-648. [ Links ]
7. Hawkey, C. J. The Lancet, 1999, 353, 307-314. [ Links ]
8. (a) Arsenijevic, L.; Arsenijevic, J.; Horeau, A., and Jacques, J. Org. Synth. 1973, 53, 5; [ Links ] (b) Chemical Abstracts 1984, 101, 151604e. [ Links ]
9. Finar, I. L.; Simmonds, A. B. J. Chem. Soc. 1958, 200-204. [ Links ]
10. Newman, M. S.; Karnes, H. A. J. Org. Chem. 1966, 31, 3980-3981. [ Links ]
11. Sandler, S. R.; Karo, W. Organic Functional Group Preparations, Academic Press, New York, 1968, p 486. [ Links ]
12. Leonard, N. J.; Johnson, C.R. J. Org. Chem. 1962, 27 , 282-284. [ Links ]
13. Donalson, R. E; Saddler, J. C. Byrn, S.; Mckenzie, A.T.; Fuchs, P. L. J. Am. Chem. Soc. 1983, 48, 2167-2188. [ Links ]
14. Cooper, J.E.; Paul, J.M. J. Org. Chem. 1970, 35, 2046-2048. [ Links ]
15. This method, developed in our laboratory, will be reported elsewhere.
16. López Muñoz, F. J.; Salazar, L. A.; Castañeda Hernández, G.; Villarreal, J .E. Drug Development Research 1993, 28, 169-175. [ Links ]














