Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de la Sociedad Química de México
Print version ISSN 0583-7693
Rev. Soc. Quím. Méx vol.48 n.1 Ciudad de México Jan./Mar. 2004
Investigación
Enantioselective Ring Opening of meso-Cyclohexene Epoxide with Phenyllithium Catalyzed by Chiral Schiff Base Ligands
Norma Aidé Cortez, Lucía Z. Flores-López, Miguel Parra-Hake, Gerardo Aguirre, Lars H. Hellberg and Ratnasamy Somanathan*
Centro de Graduados e Investigación del Instituto Tecnológico de Tijuana, Blvd. Industrial S/N, Mesa de Otay. Tijuana 22518, Baja California, México. Apdo. Postal 1166, 22000. Tel: (664) 623-3772; Fax: (664) 623-4043. E-mail: somanathan@tectijuana.mx
Recibido el 16 de febrero del 2004.
Aceptado el 31 de marzo de 2004.
Abstract
A library of chiral Schiff base ligands was synthesized from aromatic aldehydes and chiral amino alcohols. These ligands were then screened as catalysts in the asymmetric ring opening of meso-cyclohexene epoxide with phenyllithium to give (1S,2R)-phenylcyclohexanol in 9-67% ee.
Key words: Chiral, Schiff base, meso-cyclohexene epoxide, phenyllithium, ring opening.
Resumen
Se ha sintetizado una biblioteca de ligandos quirales tipo base de Schiff a partir de aldehídos aromáticos y aminoalcoholes quirales. Se midió la capacidad catalítica de estos ligandos en la apertura asimétrica del anillo de meso-epóxido de ciclohexeno con fenillitio para dar (1S,2R)-fenilciclohexanol con 9-67% ee.
Palabras clave: Quiral, base de Schiff, meso-epóxido de cyclohexeno, fenillitio, apertura de anillo.
This paper is dedicated to the memory of Dr. Raymundo Cruz Almanza
Introduction
The enantioselective ring opening of a symmetrical epoxide with different nucleophiles is an attractive and powerful method for making a large variety of chiral intermediates, which are used in the synthesis of other complex molecules [1,2], Scheme 1.
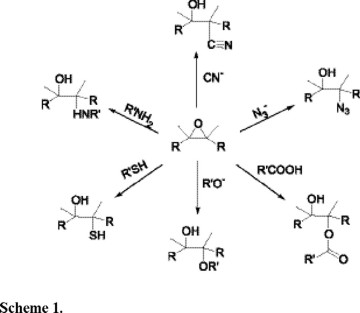
In the recent literature there are a number of reports about asymmetric ring opening of meso-epoxides leading to chiral molecules [3,4]. Jacobsen and co-workers have reported a variety of Lewis acid Salen complexes as catalysts such as Al, Ti, Cr, Mn, Fe, and Co, of which the Cr(III) Salen complex with trimethylsilyl azide gave the best enantioselectivity [3]. After azide elimination, the corresponding enone, a useful precursor in prostaglandin synthesis (Scheme 2), was obtained with 99% ee.

The same authors have used the azido alcohol obtained by asymmetrical ring opening of 1,4-cyclohexadiene monoepoxide to synthesize balanol, a natural product known to inhibit proteinkinase-C at low molar concentrations (Scheme 3) [5].

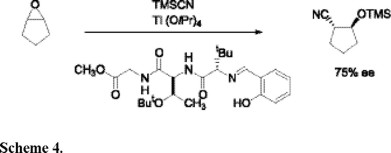
In 1998 Hoveyda introduced a peptide-based helical chiral Schiff base ligand-titanium complex as an effective catalyst for asymmetric ring opening of a meso-epoxide with trimethylsilyl cyanide (Scheme 4) [6].
In a similar reaction, Jacobsen and co-workers used (pybox)-lanthanide complexes to asymmetrically open the ring in meso-epoxides with TMSCN (Scheme 5). They reported the enantioselectivity as a function of the lanthanide atomic number, ytterbium and lutetium giving the highest selectivity [7].

Using this methodology for asymmetric ring opening of a meso-epoxide, Shibasaki et al elegantly demonstrated the total synthesis of 4-demethoxydaunomycin, a biologically active molecule. They used the intermediate amino alcohol, obtained by enantioselective opening of the meso-epoxide with the suitable amine and binol-triphenylphosphine as catalyst, which was then led through several steps to the desired molecule (Scheme 6) [8].

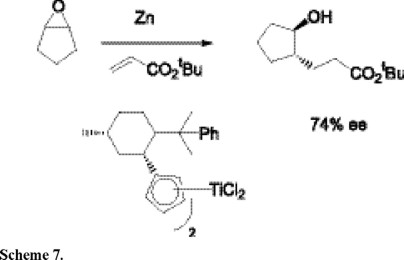
Further, Gansauer and co-workers have demonstrated that the reductive ring opening of meso-epoxides with tert-butyl acrylate to give chiral alcohols, catalyzed by a titanocene based catalyst, again represents a useful synthetic methodology leading to the synthesis of prostaglandins (Scheme 7) [9].
Other nucleophiles such as halides [10], phenoxides [11], carboxylic acids [12], thiols [13] and phenyllithium [14,15] have also been used for the asymmetric ring opening of cyclohexene epoxide. These reactions clearly illustrate the wide array of chiral catalysts that are available to carry out this reaction, providing easy access to enantiopure intermediates that can be transformed into biologically useful complex molecules. Among these studies, the reaction that attracted our attention was the asymmetric ring opening of cyclohexene epoxide with phenyllithium, catalyzed by a chiral Schiff base ligand reported by Oguni and co-workers (Scheme 8) [15].

Enantiomerically pure trans-2-phenylcyclohexanol, first used by Whitesell as a chiral auxillary [16] has become a popular reagent in a number of asymmetric transformations [17]. Some recent applications include asymmetric azoene reactions [18], [4+2]-cycloaddition reactions [19], ketene-olefin [2+2] -reactions [20], enolateimine cycloadditions [21], Pauson-Khand reactions [22], palladium annulations [23], and Reformatsky reactions [24]. Despite its potential, use of this chiral auxiliary on a preparative scale is currently limited due to lack of an efficient synthesis to this molecule. This prompted us to explore the use of structurally new chiral Schiff base ligands in the asymmetric ring opening of meso-cyclohexene epoxide with phenylithium.
Results and Discussion
As a part of a wider study of asymmetric transformations, we have synthesized a large library of non-racemic Schiff base ligands of the [ONO]-type. We have explored the use of these ligands with the transition metal ions Ti(IV), V(IV), and Cu(II) in various asymmetric chemical transformations, such as trimethylsilyl cyanation [25,26], oxidation of sulfides to sulfoxides [27] and cyclopropanation [28]. Our goal in the present study was to screen the library of Schiff base ligands as catalysts during the asymmetric ring opening of cyclohexene epoxide. In their work involving Schiff base-catalyzed ring opening of meso-epoxide Oguni et al [15 ] were not able to identify the actual catalytic species in the ring opening reaction. However, it is reasonable to assume that the active species in the reaction is a phenoxide-alkoxide-lithium aggregate, similar to the enolate aggregates proposed by Juaristi and Seebach for the lithium enolates involved in the Aldol and Michael addition reactions [29]. The interesting feature in Oguni's reaction is the enantioselectivity observed for the ring opening of the meso-epoxide to give (1S,2R)-phenylcyclo-hexanol. Based on this assumption, we hypothesized that increasing the steric bulk on the Schiff base, we might be able to create a more crowded and rigid lithium phenoxide cluster which may then enhance the enantioselectivity for the epoxide opening reaction. In other related studies using chiral Schiff base ligands for the trimethylsilyl cyanation of benzaldehyde [25, 26] and the oxidation of sulfides to sulfoxides [27], we discovered that steric crowding around the ligand enhances the observed enantioselectivity. Therefore, we presumed that a similar phenomenon may also exist in the epoxide ring opening reaction.
Our intial efforts focused on the screening of a variety of Schiff base ligands in which the substituents on the aryl aldehyde and amino alcohol fragments were varied to ascertain their effects on the reaction (Table 1). The Schiff base ligands were synthesized by an earlier reported method [25], shown in Schemes 9 and 10.
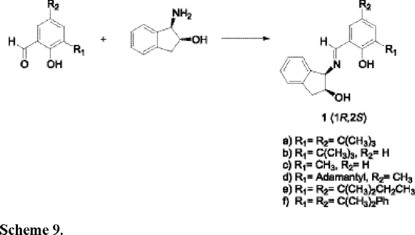

As predicted, ligands with sterically bulky groups ortho to the hydroxyl and rigid indanol derived ring on the amino alcohol fragment of the Schiff base, gave good enantioselectivity in the cyclohexene epoxide ring opening with phenyllithium to give (1S,2R)-phenylcyclohexanol in 56-66% ee (Table 1, entries 1-3) (the absolute configuration was assigned based on Oguni's ligand [15], entry 12). Similarly, having adamantyl ortho to the phenolic hydroxyl group and a tert-butyl on the amino alcohol fragment led to good enantioselectivity (entry 7). However, with large groups such as, 1,1-dimethylbenzyl, 1,1-dimethylpropyl and adamantyl on the aromatic ring and a rigid five membered cyclic system on the amino alcohol fragment, led to racemic mixtures (entries 4-6). Substituents larger than tert-butyl, probably inhibit the formation of the active lithium clusters, thus leading to racemic mixtures. Similarly, a tert-butyl group ortho to the phenolic hydroxy group, but sterically less bulky substituents on the amino alcohol fragment led to lower ee's. (entries 9-11). These results are compatible with those obtained for asymmetric trimethylsilycyanation [25,26] and sulfide oxidation reactions [27] using similar ligands. In all cases the examined ligands with the tert-butyl group tend to give the best results. Surprisingly, in our hands the ring opening reaction with Oguni's ligand (entry 12) [15], gave lower yield and low enantioselectivity (entry 13).
Conclusion
In conclusion, our Schiff base ligands gave reasonably good enantioselectivity during the ring opening of cyclohexene oxide with phenyllithium providing (1S,2R)-cyclohexanol in 9-67% ee. Unfortunately, the formation of enantiotopically favored (1S,2R)-cyclohexanol as the major product cannot be explained. It seems that the size of substituents on the aryl ring and on the amino alcohol fragments have to be modulated in order to obtain the best results. The crowding around the lithium complex probably leads to the favored estereoisomer.
Experimental
Details on the general synthesis and characterization of the Schiff bases are reported in previous work [25]. Enantiomeric excesses were determined using a HPLC with a Whelk-01 Pirkle column. HPLC conditions: solvent mixture hexane/i-PrOH (99.5/5), flow rate 1.0 mL/min, 254 nm. Retention times (min): 12 and 13 for (1S,2R) and (1R,2S), respectively, established with Oguni's ligand [15].
(1R,2S)-2-phenyl-1-cyclohexanol. In a Schlenk tube the ligand (5% mmol) in hexane (3.0 mL) was stirred under argon with phenyllitium (1.65 mmol, 1.5 mL of 1.1 M solution in cyclohexane-ethyl ether), at room temperature for 1 h. Cyclohexene oxide (0.1 mL, 1.0 mmol) was added to the solution and the resulting mixture stirred for 24 h. The reaction mixture was quenched with water and extracted with ethyl ether and analyzed by HPLC.
Acknowledgements
We gratefully acknowledge support for this project by COSNET grant 498.01-P (2001), CONACYT grant 37827-E (2001) and graduate fellowship by CONACYT for NAC.
References
1. Hodgson, D. M.; Gibbs, A. R.; Lee. G. P. Tetrahedron 1996, 52, 14361-14384. [ Links ]
2. Wills, M. C. J. Chem. Soc. Perkin Trans. 1 1999, 1765-1784. [ Links ]
3. a) Martínez, L. E.; Leighton, J. L.; Carsten, D. H.; Jacobsen, E. N. J. Am. Chem. Soc. 1995, 117, 5897-5898. [ Links ] b) Leighton, J. L.; Jacobsen, E. N. J. Org. Chem. 1996, 61, 389-390. [ Links ] c) Hansen, K. B.; Leightin, J. L.; Jacobsen, E. N. J. Am. Chem. Soc. 1996, 118, 10924-10925. [ Links ] d) Jacobsen, E. N. Acc. Chem. Res. 2000, 33, 412-431. [ Links ]
4. Yamasaki, S.; Kanai, M.; Shibasaki, M. Chem. -Eur. J. 2001, 7, 4066-4072. [ Links ]
5. Wu, M.; Jacobsen, E. N. Tetrahedron Letters 1997, 38, 1693-1696. [ Links ]
6. Shimizu, K. D.; Snapper, M. L.; Hoveyda, A. H. Chem. -Eur. J. 1998, 4, 1855-1899. [ Links ]
7. Schaus, S. E.; Jacobsen, E. N. Org. Lett. 2000, 2, 1001-1004. [ Links ]
8. Sekine, A.; Ohshima, T.; Shibasaki, M. Tetrahedron 2002, 58, 75-82. [ Links ]
9. Gansauer, A.; Lauterbach, T.; Bluhm, H.; Noltemeyer, M. Angew. Chem. Int. Ed. 1999, 38, 2909-2910. [ Links ]
10. a) Nugent, W. A. J. Am. Chem. Soc. 1998, 120, 7139-7140. [ Links ] b) McCleland, B.W.; Nugent, W.A.; Finn, M.G. J. Org. Chem. 1998, 63, 6656-6666. [ Links ] c) Denmark, S. E.; Barsanti, P. A. J. Org. Chem. 1998, 63, 2428-2429. [ Links ]
11. a) Annis, D. A.; Jacobsen, E. N. J. Am. Chem. Soc. 1999, 121, 4147-4154. [ Links ] b) Matsunga, S.; Ohshima, T.; Shibasaki, M. Adv. Synth. Catal. 2002, 344, 3-15. [ Links ]
12. Jacobsen, E. N.; Kakiuchi, F.; Konsler, R. G.; Larrow, J. F.; Tokunga, M. Tetrahedron Lett. 1997, 38, 773-776. [ Links ]
13. a) Iida, T.; Yamamoto, N.; Sasai, H.; Shibasaki, M. J. Am. Chem. Soc. 1997, 119, 4783-4784. [ Links ] b) Wu, J.; Hou, X-L; Dai, L-X; Xia, L-j; Tang, M-H. Tetrahedron: Asymmetry. 1998, 9, 3431-3436. [ Links ]
14. Mizuno, M.; Kanai, M.; Iida, A.; Tomito, K. Tetrahedron: Asymmetry 1996, 7, 2483-2484. [ Links ]
15. Oguni, N.; Miyagi, Y.; Itoh, K. Tetrahedron Lett. 1998, 39, 9023-9026. [ Links ]
16. Whitsell, J. K.; Chen, H. -H.; Lawrence, R. M. J.Org. Chem. 1985, 50, 4663-4664. [ Links ]
17. Whitsell, J. K. Chem. Rev. 1992, 92, 953-964. [ Links ]
18. Brimble, S. E.; Lee, C. K.Y. Tetrahedron: Asymmetry 1998, 9, 873-884. [ Links ]
19. Denmark, S. E.; Hurd, A. R.; Sach, H. J. J. Org. Chem. 1997, 62, 1668-1674. [ Links ]
20. De Azevedo, M. B. M.; Murta, M. M.; Greene, A. E. J. Org. Chem. 1992, 57, 4567-4569. [ Links ]
21. Ojima, I.; Habas, I.; Zhao, M.; Zucco, M.; Park, Y. H.; Sun, C. M.; Brigaud, T. Tetrahedron 1992, 48, 6985-7012. [ Links ]
22. Castro, J.; Moyano, A.; Pericãs, M. A.; Riera, A.; Greene, A. E.; Alvarez-Larena, A.; Piniella, J. F. J. Org. Chem. 1996, 61, 9016-9020. [ Links ]
23. Chassaing, C.; Haudrechy, A.; Langlois, Y. Tetrahedron Lett. 1999, 23, 8805-8809. [ Links ]
24. Shankar, B. B.; Kirkup, M. P.; McCombie, S. W.; Clader, J. W.; Ganguly, A. K. Tetrahedron Lett. 1996, 37, 4095-4098. [ Links ]
25. Z. Flores-López, L., Parra-Hake, M.; Somanathan, R.; Walsh, P. J. Organometallics 2000, 19, 2153-2160. [ Links ]
26. Gama, A.; Z. Flores-López, L.; Aguirre, G.; Parra-Hake, M.; Somanathan, R.; Walsh., P. J. Tetrahedron: Asymmetry 2002, 13, 149-154. [ Links ]
27. Gama, A.; Z. Flores-López, L.; Aguirre, G.; Parra-Hake, M.; Hellberg, L. H.; Somanathan, R. ARKIVOC 2003, XI, 4-15. [ Links ]
28. Iglesias, A.; Aguirre, G.; Somanathan, R.; Parra-Hake, M. Polyhedron. Submitted. [ Links ]
29. Juaristi, E.; Beck, A. K.; Hansen, J.; Matt, T.; Mukhopadhyay, T; Simson, M.; Seebach, D. Synthesis 1993, 1271- 1290. [ Links ]














