Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Sociedad Química de México
versión impresa ISSN 0583-7693
Rev. Soc. Quím. Méx vol.48 no.1 Ciudad de México ene./mar. 2004
Investigación
Synthesis of Miconazole and Analogs Through a Carbenoid Intermediate
Erick Cuevas Yañez,1* Adriana Canul Sánchez,1 Juan Manuel Serrano Becerra,2 Joseph M. Muchowski3 and Raymundo Cruz Almanza1†
1 Instituto de Química de la Universidad Nacional Autónoma de México. Circuito Exterior, Ciudad Universitaria, Coyoacán, 04510, México, D.F. Tel. (52) 56 22 44 08; fax (52) 56 16 22 17. E-mail: erick.cuevas@correo.unam.mx
2 Facultad de Química de la Universidad Autónoma del Estado de México. Paseo Colón y Paseo Tollocan, Toluca, 50000, Toluca, Estado de México.
3 Chemistry, Roche Palo Alto, 3431 Hillview Avenue, Palo Alto, CA 94304-1320, USA.
Recibido 27 de febrero del 2004.
Aceptado el 31 de marzo del 2004.
Abstract
An alternative synthesis for the preparation of miconazole, enilconazole and econazole is described. The process involves the intermolecular insertion of a carbenoid species to imidazole from α-diazoketones with copper acetylacetonate as the key reaction of the synthesis route.
Keywords: Imidazole, carbenoid, miconazole.
Resumen
Se describe una síntesis alternativa para la obtención de miconazol, enilconazol y econazol. El proceso involucra la inserción intermolecular de una especie carbenoide a imidazol a partir de α-diazocetonas con acetilacetonato de cobre como reacción clave de la ruta de síntesis.
Palabras clave: Imidazol, carbenoide, miconazol.
Introduction
The carbenoids derived from diazoketones have been effective intermediates in organic syntheses, specially the reaction of carbenoids over rich-electron heterocyclic compounds in the synthesis of polyenes from furans [1], and in the indolizine alkaloids synthesis from pyrroles [2]. Additionally carbenoid intermolecular and intramolecular insertions have been reported in thiophene [3], indole [4] and benzofuran [5].
In contrast, two-heteroatom five membered rings have been less studied in this area. Thiazoline ring in some penicillin derivatives reacted with an excess of ethyl diazoacetate in the presence of catalytic copper acetylacetonate to give [3+2] cycloaddition aducts [6]. On the other hand, an intramolecular cyclization through a carbenoid insertion occurred when 3-isoxalyl diazobutanone reacted with catalytic of rhodium (II) acetate [7].
At the moment, few studies on carbenoid insertions on imidazoles have been done. Pellicciari and coworkers [8] reported the imidazole alkylation from α-diazocarbonyl compounds and copper bronze in moderate yields, however, they demonstrated that carbenoid insertions on imidazoles are feasible processes and that could be used in the synthesis of some imidazole pharmaceutical known products.
For example, miconazole (6) has a broad-spectrum antifungal activity in vitro and its therapeutic use in the treatment of dermatophytic infections is well known [9]. A number of publications for the miconazole synthesis has been reported [10-12].
These elements motivated us to develop an alternative synthetic route to prepare miconazole where the key step could be a carbenoid insertion to imidazole.
Results and discussion
Initially, we attempted to prepare the 2,4-dichlorodiazoacetophenone 1 from 2,4-dichlorobenzoic acid using a novel technology that involves the use of acyloxyphosphonium salts [13]. Thus, the treatment of equimolar amounts of 2,4-dichlorobenzoic acid, triphenylphosphine and a slight excess of N-bromosuccinimide in THF at 0 °C during 15 min, and the subsequent addition of a excess of ethereal diazomethane (5: 1) gave the corresponding diazoketone 1 in 76 % yield.
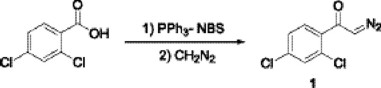
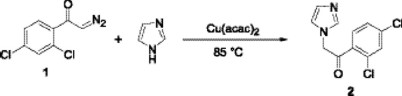
Diazoketone 1 was reacted with imidazole and a catalytic amount of copper (II) acetylacetonate (10% mol) in toluene at 85 °C, in a modification of the procedures described by the Sweeney [14] and West's [15] groups, to obtain the imidazolyl ketone 2 in 46 % yield. Although copper bronzes were used in the carbenoid insertions on imidazoles, other copper salts have not been studied yet. The use of Cu(acac)2 decreases the temperature reaction and optimizes the process, other products are not detected while this procedure occurs in mild conditions and it is broad in scope.
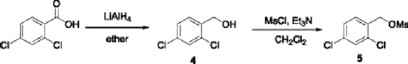
The imidazolyl ketone 2 was reduced to alcohol 3 using sodium borohydride in methanol, this reaction has been reported by Godefroi et al [10].

On the other hand, 2,4-dichlorobenzoic acid was treated with lithium aluminum hydride to obtain the benzyl alcohol 4 which in turn was reacted with methanesulfonyl chloride to obtain the mesylate 5.
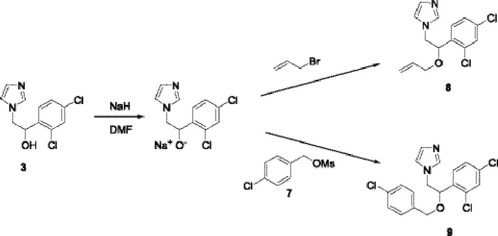
Finally, the sodium salt from alcohol 3 was mixed with the crude mesylate 5 in DMF at room temperature, and after work up, miconazole (6) was obtained in 70 % yield.
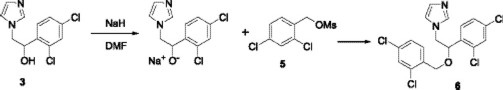
Using similar conditions, we prepared enilconazole 8 [16] and econazole 9 from the sodium salt of alcohol 3 with allyl bromide and the mesylate 7 respectively.
In summary, miconazole, econazole and enilconazole were prepared using α-diazocarbonyl compounds as departure materials. Novel imidazoles with antifungal activity can be obtained by this procedure. The carbenoid insertions are an excellent alternative route to prepare N-alkylated imidazoles, and some examples of their synthetic versatility were presented here. We hope to extend this method to the synthesis of other important imidazole products.
Experimental
General procedures were described previously [17].
2-Diazo-1-(2,4-dichlorophenyl)-ethanone (1). To a solution of PPh3 (0. 262g, 1 mmol) and 2,4-dichlorobenzoic acid (0.191g, 1 mmol) in anhydrous THF (1 mL) at 0 °C, NBS (0.182 g, 1.1 mmol) in THF (5 mL) was added dropwise over a 10 min period under a nitrogen atmosphere. The resulting reaction mixture was allowed to warm to room temperature continuing the stirring for an additional 15 min. The mixture was cooled to 0 °C again. Then, an ether solution of diazomethane (5 mmol) from N-methyl-N-nitroso-4-toluenesulfonamide (7.15 mmol) was added. A vigorous evolution of nitrogen occurred, and the mixture was allowed to warm to room temperature overnight. The solvent was removed in vacuo and the product was purified by flash column chromatography (SiO2, hexane/ethyl acetate 9:1) to afford the compound 1 as a yellow oil (0.163 g, 76%). IR (CHCl3) νmax 2110, 1620, 1354 cm-1; 1H NMR (CDCl3, 200 MHz) δ 5.82 (s, 1H), 7.40-7.85 (m, 3H); 13C NMR (CDCl3, 50 MHz) δ 57.5, 126.9, 129.2, 131.4, 135.3, 136.4, 139.6, 194.7; MS [EI+] m/z (%): 215 [M]+ (85), 187 [M- N2]+ (38), 174 [M - CHN2]+(55), 146 [M - COCHN2]+ (100).
1-(2,4-Dichlorophenyl)-2-imidazol-1-ylethanone (2). To a stirring solution of imidazole (0.136 g, 2 mmol) and Cu(acac)2 ( 0.048 g, 0.2 mmol) in toluene ( 2 mL) at 85 °C was added a solution of diazoketone 1 (0.45 g, 1.05 mmol) in toluene (8 mL) via syringe pump over 1 h under a nitrogen atmosphere. The resulting mixture was allowed to cool to room temperature continuing the stirring for additional 1 h. The solvent was removed in vacuo and the product was purified by flash column chromatography (SiO2, CH2Cl2 /methanol 95:5) to afford the compound 1 as a white solid (0.234 g, 46%), mp 169-170 °C; IR (CHCl3) νmax 2929, 1715, 1584 cm-1; 1H NMR (DMSO-d6, 200 MHz) δ 5.03 (s, 2H), 6.92 (s, 1H), 7.04 (s, 1H), 7.30 (m, 1H), 7.43 (m, 1H), 7.49 (s, 1H), 7.78 (m, 1H); 13C NMR (DMSO-d6, 50 MHz) δ 58.3, 122.6, 125.9, 126.9, 129.2, 131.4, 135.3, 139.6, 139.8, 194.7; MS [FAB+] m/z (%): 255 [M + 1]+ (86).
1-(2,4-Dichlorophenyl)-2-imidazol-1-ylethanol (3). To a suspension of sodium borohydride (0.045 g, 1.1 mmol) in anhydrous methanol (50 mL) was added a solution of compound 2 (0.284 g, 1.1 mmol) in methanol (5 mL) maintaining the temperature below 5 °C under a nitrogen atmosphere. The resulting mixture was stirred at room temperature for 1 h and thereafter was heated at 50 °C for 1 h. The reaction was cooled to room temperature and the solvent was removed in vacuo. Water (10 mL) was added and the product was extracted with ethyl acetate (3 × 5 mL), the organic phase was dried over Na2SO4 and the solvent was removed in vacuo to yield a white solid (0.172g, 60%) which was purified by crystallization, mp 131-133 °C; IR (CHCl3) νmax 3318, 2927, 1588 cm-1; 1H NMR (CDCl3, 200 MHz) δ 3.92( dd, 1H; JAB = 15 Hz and JAX = 9 Hz), 4.24 (dd, 1H, JBA = 15 Hz and JBX = 5 Hz), 5.24 (dd, 1H, JXA = 9 Hz and JXB = 5 Hz), 5.83 (s, 1H,), 6.88-7.86 (m, 6H); 13C NMR (CDCl3, 50 MHz) δ 57.6, 67.6, 120.3, 127.8, 128.8, 130.0, 130.1, 131.6, 133.5, 138.7, 139.8; MS [FAB+] m/z (%): 257 [M + 1]+ (30).
2,4-Dichlorophenylmethanol (4). To a solution of 2,4-dichlorobenzoic acid (0.5 g, 2.6 mmol) in anhydrous ether (5 mL) was added dropwise a suspension of lithium aluminum hydride (0.124 g, 3.2 mmol) in ether (10 mL) at 0 °C under a nitrogen atmosphere. The resulting mixture was heated at reflux temperature during 50 min. The reaction was cooled to 0 °C, water (5 mL) was added and the mixture was acidified to pH = 4 with 10% HCl solution. The product was extracted with CH2Cl2 (3 x 25 mL), the organic phase was dried over Na2SO4 and the solvent was removed in vacuo to afford the compound 4 in quantitative yield as a colorless oil which was used without purification. IR (CHCl3) νmax 3235, 2919, 1591 cm-1; 1H NMR (CDCl3, 200 MHz) δ 4.74 ( s, 2H ), 7.24 ( dd, 1H, Jortho = 8.2 Hz, Jmeta= 2.0 Hz ), 7.37 ( d, 1H, Jmeta= 2 Hz ), 7.43 ( d, 1H, Jortho = 8.2 Hz ); 13C NMR (CDCl3, 50 MHz) δ 62.1, 127.2, 129.1, 129.3, 133.1, 133.6, 136.7; MS [FAB+] m/z (%): 177 [M + 1]+ (15).
Preparation of Miconazole, 1-[2-(2,4-dichlorobenzyloxy)-2-(2,4-dichlorophenyl)ethyl] imidazole (6). 2,4-dichlorophenylmethanol 4 (0.212 g, 1.2 mmol) was dissolved in CH2Cl2 (10 mL) and cooled at 0 °C. Freshly distilled Et3N (0.17 mL, 1.2 mmol) was added to this solution, followed by dropwise addition of methanesulfonyl chloride (0.094 mL, 1.2 mmol). After addition was complete, stirring was continued at 0 °C under a nitrogen atmosphere for an additional 90 min and the solvent was removed in vacuo.
In a separate flask, a suspension of sodium hydride (0.040 g, 1.1 mmol) in DMF (5 mL) was treated with a solution of alcohol 3 (0.256 g, 1 mmol) in DMF (10 mL) at 0 °C, the resulting mixture was stirred under a nitrogen atmosphere at room temperature for 1 h. The mixture was cooled at 0 °C and a solution of the crude mesylate in DMF (5 mL) was added. The resulting mixture was stirred for 1 h at room temperature. The reaction was quenched by addition of water (150 mL). The aqueous phase was extracted with ethyl acetate (3 x 25 mL), the organic phase was washed with water (150 mL), dried over Na2SO4 and the solvent was removed in vacuo. Purification by column chromatography (SiO2, hexane/ethyl acetate 7:3) yielded a white solid (0.28 g, 70%), mp 184-185 °C; IR (CHCl3) νmax 2968, 1590 cm-1; 1H NMR (CDCl3, 200 MHz) δ 4.01 (m, 2H), 4.09 (dd, 1 H, JAB = 14 Hz y JAX = 8 Hz), 4.29 (dd, 1H, JBA = 14 Hz y JBX = 5 Hz), 4.82 (dd, 1 H, JXA = 8 Hz y JXB = 5 Hz), 6.77 (m, 2H), 7.12 (m, 6H), 7.32 (m,1H); 13C NMR (CDCl3, 50 MHz) δ 57.6, 62.1, 67.6, 120.3, 127.2, 127.8, 128.8, 129.1, 129.3, 130.0, 130.1, 131.6, 133.1, 133.5, 133.6, 136.6, 138.7, 139.8; MS [FAB+] m/z (%): 415 [M + 1]+ (20).
Preparation of Enilconazole, 1-[2-allyloxy-2-(2,4-dichlorophenyl)ethyl]imidazole (8). A suspension of sodium hydride (0.040 g, 1.1 mmol) in DMF (5 mL) was treated with a solution of alcohol 3 (0.256 g, 1 mmol) in DMF (10 mL) at 0 °C. The resulting mixture was stirred under a nitrogen atmosphere at room temperature for 1 h. The mixture was cooled at 0 °C and allyl bromide (0.1 mL, 1.2 mmol) was added. The resulting mixture was stirred for 1 h at room temperature. The reaction was quenched by addition of water (150 mL). The aqueous phase was extracted with ethyl acetate (3 × 25 mL), the organic phase was washed with water (150 mL), dried over Na2SO4 and the solvent was removed in vacuo. Purification by column chromatography (SiO2, hexane/ethyl acetate 7:3) afforded a colorless oil (0.24 g, 81%); IR (film) νmax 3083, 1646, 1588 cm-1; 1H NMR (CDCl3, 200 MHz) δ 3.97 (m, 2H), 4.05 (dd, 1H, JAB = 16 Hz and JAX = 8 Hz), 4.25 (dd, 1H, JBA = 16 Hz and JBX = 4 Hz), 4.93 (m, 2H), 5.19 (dd, 1H, JXA = 8 Hz and JXB = 4 Hz), 5.66-5.82 (m, 1H), 7.03-7.81 (m, 3H); MS [FAB+] m/z (%): 297 [M + 1]+ (26).
4-Chlorophenylmethanol. The procedure was similar to that used in the preparation of 2,4-dichlorophenyl methanol (4) affording a colorless oil (98%); IR (CHCl3) νmax 3230, 2919, 1590 cm-1; 1H NMR (CDCl3, 200 MHz) δ 4.59, 7.18 (d, 2H), 7.36 (d, 1H); MS [FAB+] m/z (%): 143 [M + 1]+ (25).
Preparation of econazole, 1-[2-(4-chlorobenzyloxy)-2-(2,4-dichlorophenyl)]imidazole (9). The procedure was similar to that used in the preparation of miconazole, yielding a white solid (75 %), mp 166 °C; IR (CHCl3) νmax 3067, 2969, 1594 cm-1; 1H NMR (CDCl3, 200 MHz) δ 4.05 (m, 2H), 4.15 (dd, 1H, JAB = 14 Hz y JAX = 8 Hz), 4.35 (dd, 1H, JBA = 14 Hz y JBX = 5 Hz), 4.88 (dd, 1H, JXA = 8 Hz y JXB = 5 Hz), 6.89 (m, 2H), 7.18 (m, 5H), 7.36 (m, 2H); MS [FAB+] m/z (%): 381 [M + 1]+ (5).
Acknowledgments
Financial support from CONACyT (no. 37312-E) is gratefully acknowledged. The authors would like to thank Rocío Patiño, Angeles Peña, Elizabeth Huerta, Nieves Zavala, Francisco Javier Pérez Flores and Luis Velasco for their technical support.
References
1. (a) Wenkert, E.; Guo, M.; Lavilla, R.; Porter, B.; Ramachandran, K.; Sheu, J. H. J. Org. Chem. 1990, 55, 6203-6214. [ Links ] (b) Wenkert, E.; Decorzant R.; Naf, F. Helv. Chim. Acta 1989, 72, 756-766. [ Links ] (c) Wenkert, E.; Guo, M.; Pizzo, F.; Ramachandran, K. Helv. Chim. Acta 1987, 70, 1429-1438. [ Links ]
2. (a) Jefford, C. W.; Wang, J. B.; Tetrahedron Lett. 1993, 34, 3119-3122. [ Links ] (b) Jefford, C. W.; Tang, Q.; Zaslona , A. J. Am. Chem. Soc. 1991, 113, 3513-3518. [ Links ] (c) Jefford, C. W.; Tang, Q.; Zaslona, A. Helv. Chim. Acta 1989, 72, 1749-1752. [ Links ] (d) Jefford, C. W.; Kubota, T.; Zaslona, A. Helv. Chim. Acta 1986, 69, 2048-2061. [ Links ]
3. (a) Yong, K.; Salim, M.; Carpeta A. J. Org. Chem. 1998, 63, 9828-9833. [ Links ] (b) Trammer, G. K.; Capretta, A. Tetrahedron 1998, 54, 15499-15508. [ Links ] (c) Frampton, C. S.; Pole, D. L.; Yong, K.; Capretta, A. Tetrahedron Lett. 1997, 38, 5081-5084. [ Links ] (d) Stortlor, H.; Skramstad, J.; Nordenson, S. J. Chem. Soc. Chem Comm. 1984, 208-209. [ Links ]
4. (a) Gibe, R.; Kerr, M. A. J. Org. Chem. 2002, 67, 6247-6249. [ Links ] (b) Muthusamy, S.; Gunanathan, C., Arulananda, S.; Suresh, E.; Dastidar, P. J. Chem Soc. Chem. Comm. 2002, 824-825. [ Links ] (c) Salim, M.; Capretta, A. Tetrahedron 2000, 56, 8063-8069. [ Links ] (d) Matsumoto, M.; Watanabe, N.; Kobayashi, H. Heterocycles 1987, 26, 1479-1482. [ Links ]
5. (a) Padwa, A., Wisnieff, T. J.; Walsh, E. J. J. Org. Chem. 1986, 51, 5036-5038. [ Links ] (b) Padwa, A.; Wisnieff T. J.; Walsh, E. J. J. Org. Chem. 1989, 54, 299-308. [ Links ]
6. Mara, A. M.; Singh, O.; Thomas, E. J.; Williams D. J. J. Chem Soc. Perkin Trans. 1 1982, 2169-2173. [ Links ]
7. Padwa, A.; Dean, D. C.; Osterhout, M. H.; Precedo, L.; Semones M. A. J. Org. Chem. 1994, 59, 5347-5357. [ Links ]
8. (a) Pellicciari, R.; Curini, M.; Spagnoli, N.; Ceccherelli, P. Synthesis 1981, 629-631. [ Links ] (b) Pellicciari, R.; Curini, M.; Spagnoli, N. Archiv. Pharm. 1984, 317, 38-41. [ Links ]
9. (a) Kobayashi, G. S.; Medoff, G. Ann. Rev. Microb. 1977, 31, 291-308. [ Links ] (b) Heel, R.G.; Broaden, R. N.; Pakes, G. E.; Speight, T. M.; Avery G.S. Drugs 1980, 11, 7-24. [ Links ]
10. Godefroi, E. F.; Heeres, J.; Cutsem, J. V.; Janssen, P. A. J. J Med. Chem. 1969, 12, 784-791. [ Links ]
11. (a) Godefroi, E.; Heeres, J. Ger. Offen. 1970 DE 1940388 19700226, Chem. Abs. 1970, 72, 90466. [ Links ] (b) Molina-Caprile, F. Spanish Patent 1983 ES 510870, Chem. Abs. 1983, 99, 105250. [ Links ] (c) Trubistsin, A. J.; Pevzner, M. S.; Kirpenk, Z. V. Russian Patent 1995 RU2043342 Cl 199550910, Chem. Abs. 1996, 124, 317159. [ Links ] (d) Liao, Y. W.; Li, H. X. Yaoxue Xuebao 1993, 28, 22; [ Links ] Chem. Abs. 1993, 118, 254361. [ Links ]
12. Tortolani, D. R.; Biller, s. A. Tetrahedron Lett. 1996, 37, 5687-5690. [ Links ]
13. Cuevas-Yañez, E.; García, M. A.; de la Mora, M. A.; Muchowski, J. M.; Cruz-Almanza, R. Tetrahedron Lett. 2003, 44, 4815-4817. [ Links ]
14. Heath, P.; Roberts, E.; Sweeney, J. B.; Wessel, H. P.; Workman, J. A. J. Org. Chem. 2003, 68, 4083-4086. [ Links ]
15. Vanecko, J. A.; West, F. G. Org. Lett. 2002, 4, 2813-2816. [ Links ]
16. Godefroi, E.F.; Schuermans, J. L.; (Janssen Pharmaceutica, N. V.) Ger. Offen. 2,063857 (Cl. C 07d) 22 Jul. 1971, US Appl. Jan 1970, US Patent 3,658,813 (1972). Chem. Abs. 1971, 75, 118319n. [ Links ]
17. Cuevas-Yañez, E.; Cruz-Almanza, R. Rev. Soc. Quím. Méx. 2004, 48, 46-48. [ Links ]
Nota
† Deceased, October 2003.














