Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Sociedad Química de México
versión impresa ISSN 0583-7693
Rev. Soc. Quím. Méx vol.47 no.2 Ciudad de México abr./jun. 2003
Investigación
The Use of N,N'-Di[α-phenylethyl]-diamines as Phosphorylated Chiral Derivatizing Agents for the Determination of the Enantiomeric Purity of Chiral Secondary Alcohols
Gloria E. Moreno,1,2 Virginia M. Mastranzo,1,2 Leticia Quintero,2 Cecilia Anaya de Parrodi,*,1 and Eusebio Juaristi*, 3
1 Centro de Investigaciones Químico Biológicas, Universidad de las Américas-Puebla, Santa Catarina Mártir, Cholula, 72820 Puebla, México. E-mail: anaya@mail.pue.udlap.mx
2 Centro de Investigación de la Facultad de Ciencias Químicas, Universidad Autónoma de Puebla, 72570 Puebla, México.
3 Departamento de Química, Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional, 07000 México, D. F. E-mail: juaristi@relaq.mx
Recibido el 17 de febrero del 2003.
Aceptado el 26 de marzo del 2003.
This paper is dedicated to Dr. Alfonso Romo de Vivar in appreciation of his contributions to chemistry.
Abstract
The influence of the framework structure in C2-symmetric N,N'-di[α-phenylethyl]diamines as chiral derivatizing agents, on their effectiveness for the determination of the enantiomeric composition of chiral secondary alcohols by 31P-NMR spectroscopy of derived diastereomeric phosphonamides, is described.
Key words: chiral derivatizing agents, enantiomeric composition, C2-symmetric diamines, 31P-NMR spectroscopy.
Resumen
Se describe la influencia de la estructura de N,N'-di[α-feniletil]-diaminas con eje de simetría C2, como agentes derivatizantes quirales en la determinación de la composición enantiomérica de alcoholes secundarios quirales empleando RMN de 31P.
Palabras clave: Agente derivatizante quiral, composición enantiomérica, diaminas con simetría C2, RMN de 31P.
Introduction
Chiral derivatizing agents (CDAs) for NMR spectroscopy represent one of the most effective tools to satisfy the great demand for rapid and reliable methods for the determination of the enantiomeric composition of chiral substrates [1]. Their use involves the derivatization reaction of an enantiomerically pure chiral auxiliary with the substrates to be analyzed, in order to obtain diastereoisomeric products presenting anisochronous absorptions in their NMR spectra. Efforts directed to the development of fast and reliable CDAs continue [2]. In this context, the high sensitivity afforded by 31P NMR spectroscopic methods makes attractive the use of phosphorus-containing derivatives towards this goal [3].
Chiral diamines have been shown to be useful chiral reagents and ligands for chemical catalysis, with especial application in asymmetric synthesis [4]. By the same token, (R)- and (S)-α-phenylethylamine are simple, yet powerful stereodifferentiating auxiliaries in organic transformations [5].
Presently, there exist several reports in the literature describing the use of diamines containing diverse N-substituents as CDAs, which are based on the synthesis of chiral phospholidines for the determination of enantiomeric composition of chiral alcohols, amines, carboxylic acids, halohydrins and thiols. In particular, N,N'-di[(S)-α-phenylethyl]ethane-1,2-diamine, A, and N,N'-di[(S)-α-phenylethyl]propane-1,3-diamine, B, (Fig. 1) have been reported as effective and inexpensive CDAs [6]. In addition, the use of chiral diamines based on enantiopure trans-1,2-diaminocyclohexane as CDAs, such as C has been demonstrated [7]. Furthermore, our group also reported the use of trans-N,N'-di-[(S)-α-phenylethyl]-cyclohexane-1,2-diamines, D and E as convenient CDAs [8].

Results and discussion
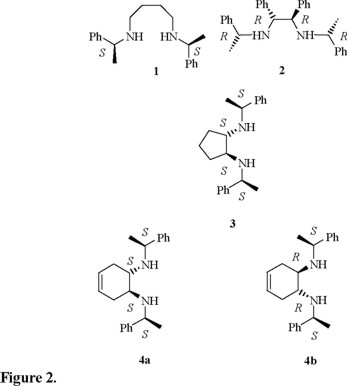
The use of C2-symmetric N,N'-di[α-phenylethyl]-diamines 1, 2, 3, 4a and 4b as chiral ligands (Fig. 2) will be reported [9]. In the present work their application as chiral derivatizing agents (CDAs), via the formation of the corresponding P-chloro-1,3-diazaphospholidines is described. These CDAs can be prepared directly in the NMR tube employed for 31P NMR analysis [8].
To this end, each diamine in CDCl3 solvent was treated with one equivalent of PCl3 in CH2Cl2 at room temperature to give the corresponding phospholidines as intermediates. The formation of the P-chloro-1,3-diazaphospholidines of interest was very fast and quantitative with diamines 1, 2, 4a, and 4b, but rather slow and incomplete with diamine 3 (Table 1). Apparently, the reaction of diamine 3 with PCl3 is also unfavorable, because of strain in the trans-fused five-membered bicyclic phospholidine formed.

Gratifyingly, the P-Cl bond of the phospholidines 2, 4a and 4b were readily cleaved, upon treatment with the carbinols of interest. So, derivatization was carried out by addition of 0.8 equiv of the racemic secondary alcohols, 5a-d, to afford the respective P-alkoxy-1,3-diazaphosphonamides (Table 2). The all-(S) diamine 4a afforded the largest differences of chemical shifts (Δδ) in the 31P NMR spectra of the diastereomeric phosphonamides. Nevertheless, the P-Cl bond of the P-chloro-1,3-diazaphospholidine derived from 1 was specially resistant upon treatment with carbinols. Thus, diamines 1 and 3 were not useful as CDAs.
In summary, the P-chloro-1,3-diazaphospholidines derived from chiral diamines 2, 4a, and 4b, incorporating N-(α-phenylethyl) substituents, are convenient chiral derivatizing agents for the determination of the enantiomeric purity of chiral alcohols. The quantitative and fast P-Cl bond cleavage upon alcoholysis leads to phosphonamide formation, directly in the NMR tube prior to measurement. Large differences in the 31P NMR chemical shifts (Δδ) for the diastereomeric phosphonamides were observed, allowing accurate integration and quantitative determination of the diastereomeric ratios.
Experimental section
31P NMR spectra were measured on a Varian Mercury-200 MHz spectrometer. Chemical shifts are given as δ values (ppm). All reagents were purchased from Aldrich Chemical Co.
General Procedure for Chiral Alcohol Derivatization
In an NMR tube are placed with vigorous stirring 0.16 mmol of free diamine, 0.5 mL of CDCl3, 119 mg (0.80 mmol) of diethylaniline, and 23 mg (0.16 mmol) of PCl3 previously dissolved in 50 µL of CH2Cl2, affording the chlorodiazaphospholidine and the 31P NMR spectra are recorded (Table I). Immediately after, 0.13 mmol of racemic secondary alcohols is added, and the resulting mixture is stirred for 30 minutes before 31P NMR spectra are recorded at room temperature (Table 2).
Acknowledgment
We thank CONACyT for financial support (Projects No. 32202-E and 33023-E, and Grants No. 91275 and 144937).
References
1. (a) For a detailed review, see: Parker, D. Chem. Rev. 1991, 91, 1441-1457. [ Links ] See, also: (b) Dale, J. A.; Mosher, H. S. J. Am. Chem. Soc. 1973, 95, 512-519. [ Links ] (c) Pirkle, W. H.; Hoover, D. J. Top. Stereochem. 1982, 13, 263-331. [ Links ] (d) Benson, S. C.; Cai, P.; Colon, M.; Haiza, M. A.; Tokles, M.; Snyder, J. K. J. Org. Chem. 1988, 53, 5335-5341. [ Links ] (e) Jursic, B. S.; Zdravkovski, Z.; Zuanic, M. Tetrahedron: Asymmetry 1995, 5, 1711-1716. [ Links ]
2. (a) Uccello-Barreta, G.; Bernardini, R.; Lazzaroni, R.; Salvadori, P. Org. Lett. 2000, 2, 1795-1798. [ Links ] (b) Reymond, S.; Brunel, J. M.; Buono, G. Tetrahedron: Asymmetry 2000, 11, 1273-1278. [ Links ] (c) Alexakis, A.; Chauvin, A.-S. Tetrahedron: Asymmetry 2001, 12, 1411-1416. [ Links ]
3. (a) Verkade, L. D.; Quin, L. D. Phosphorus-31 NMR Spectroscopy in Stereochemical Analysis; VCH Publishers: Deerfield Beach, 1987. [ Links ] (b) See, also: Juaristi, E. Introduction to Stereochemistry and Conformational Analysis; Wiley: New York, 1991; pp 137-138. [ Links ] (c) Johnson, C. R.; Elliott, R. C.; Penning, T. D. J. Am. Chem. Soc. 1984, 106, 5019-5020. [ Links ] (d) Kato, N. J. Am. Chem. Soc. 1990, 112, 254-257. [ Links ] (e) Anderson, R. C.; Shapiro, M. J. J. Org. Chem. 1984, 49, 1304-1305. [ Links ] f) Hulst, R.; Kellogg, R. M.; Feringa, B. L. Rec. Trav. Chim. Pays-Bas 1995, 114, 115-138. [ Links ]
4. For excellent reviews, see: (a) Togni, A.; Venanzi, L. M. Angew. Chem., Int. Ed. 1994, 33, 497-526. [ Links ] (b) Bennani, Y. L.; Hanessian, S. Chem. Rev. 1997, 97, 3161. [ Links ] (c) Lucet, D.; Le Gall, T.; Miokowski, Ch. Angewandte Chem., Int. Ed. 1998, 37, 2580-2627. [ Links ]
5. (a) Jaen, J. In Encyclopedia of Reagents for Organic Synthesis, Paquette, L. A., Ed.; Wiley: New York, 1995, Vol. 5, pp 3427-3431. [ Links ] (b) Juaristi, E.; Escalante, J.; León-Romo, J. L.; Reyes, A. Tetrahedron: Asymmetry 1998, 9, 715-740. [ Links ] (c) Juaristi, E.; León-Romo, J. L.; Reyes, A.; Escalante, J. Tetrahedron: Asymmetry 1999, 10, 2441-2495. [ Links ]
6. Hulst, R.; de Vries, K.; Feringa, B. L. Tetrahedron: Asymmetry 1994, 5, 699-708. [ Links ]
7. (a) Alexakis, A.; Mutti, S.; Mangeney, P. J. Org. Chem. 1992, 57, 1224-1237. [ Links ] (b) Alexakis, A.; Frutos, J. C.; Mutti, S.; Mangeney, P. J. Org. Chem. 1994, 59, 3326-3334. [ Links ] For other chiral derivatizing agents based on enantiopure trans-1,2-diaminocyclohexane, see: (c) Resch, J. F.; Meinwald, J. Tetrahedron Lett. 1981, 22, 3159-3162. [ Links ] (d) Staubach, B.; Buddrus, J. Angew. Chem., Int. Ed. 1996, 35, 1344-1346. [ Links ]
8. Anaya de Parrodi, C.; Moreno, G. E.; Quintero L.; Juaristi, E. Tetrahedron: Asymmetry 1998, 9, 2093-2099. [ Links ]
9. Mastranzo, V. M.; Quintero, L.; Anaya de Parrodi, C.; Juaristi, E.; Walsh, P. J. Unpublished results.














