Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Sociedad Química de México
versión impresa ISSN 0583-7693
Rev. Soc. Quím. Méx vol.47 no.1 Ciudad de México ene./mar. 2003
Comunicación Técnica
Effect of the mixing method on the sustained release profile of pelanserin from HPMC / citric acid matrix tablets
Rogelio Espinoza-Ramos y Leopoldo Villafuerte-Robles*
Departamento de Farmacia, ENCB-Instituto Politécnico Nacional, Carpio y Plan de Ayala s/n, C. P. 11340, D. F., México. Fax: (52) 5396-3503. E-mail: lvillaro@bios.encb.ipn.mx.
Recibido el 9 de septiembre del 2002.
Aceptado el 12 de febrero del 2003.
Resumen
Matrices con cantidades variables de ácido cítrico, obtenidas por granulación húmeda y aplicando el modelo: Mt / Minf = k tn, mostraron constantes de liberación (kprom. = 0.236) y valores del exponente n, indicativo del mecanismo de liberación (nprom. = 0.736), mayores que las obtenidas por mezclado en seco (mezclado por volteo), kprom. = 0.185 y nprom. = 0.606. Esto indica una mayor tendencia hacia un mecanismo controlado por relajación/erosión en tabletas obtenidas por granulación húmeda, que aquellas obtenidas por mezclado en seco. Los resultados se atribuyen a cambios en la distribución del ácido cítrico.
Palabras clave: Pelanserina, HPMC, Método de mezclado, Liberación sostenida, Ácido cítrico.
Abstract
Matrices obtained by wet granulation, containing variable citric acid quantities and employing the model: Mt / Minf = k tn, showed greater release constants (kav = 0.236) and exponent n values (nav = 0.736) than those obtained by mixing in the dry (tumbling mixing), kav = 0.185 and nav = 0.606. This indicates a greater tendency to a relaxation/erosion release mechanism of wet granulation compared to dry mixing. The results are attributed to changes in the citric acid distribution.
Key words: Pelanserin, HPMC, Mixing method, Sustained release, Citric acid.
Introduction
Hydroxypropyl methylcellulose (HPMC) is the leading hydrophilic carrier material used for the preparation of oral controlled drug delivery systems. The special pharmaceutical interest in hydrophilic matrices like HPMC is considered due to the possibility of a proper control of the manufacturing process, reproducible release profiles and variability slightly lower than that characterizing coated release forms. Its manufacturing process can be done via direct compression or compression with a previous granulation, either in the dry or in the wet.
The mechanisms by which HPMC retard drug release include its ability to form rapidly a gel layer at the matrix periphery exposed to aqueous fluids. The drug is released from the matrix mainly by diffusion through water filled pores. Hence, the release rate is associated to the porosity and tortuosity of the pores or channels network. The porosity and tortuosity of a swellable matrix are primarily attributed to the polymer swellability [1].
Variables such as the particle size, viscosity and proportion of HPMC modify the characteristics of porosity and tortuosity of the swollen matrix and hence, modulate the release rate of drugs. Increasing proportions of HPMC in the matrix decreases the release rate [2, 3]. An increasing particle size of HPMC produces increasing release rates from the matrix tablets [4, 5] and decreasing release rates occur often with an increasing viscosity grade [4, 6].
The drug release from matrix tablets can be modified by interactions between the polymer forming the matrix and other components of the formula such as admixed excipients and drugs [7, 8, 9, 10]. Admixing another polymer may bring about different effects according to the type and strength of the interactions between the polymers forming the gel barrier [11, 12].
As mentioned above, there have been a number of studies about the influence of formulation factors on in vitro drug release and in vivo performance of hydrophilic matrices. However, processing properties of sustained release systems based on hydrophilic matrices have been rarely reported. Traditionally wet granulation has been preferred as the processing route for hydrophilic sustained release matrix tablets in industrial production, to ensure good content uniformity and to avoid powder flow related inter-tablet weight variation problems.
It has been considered in many cases that the fabrication technique does not affect, in an important manner, the release profiles from hydrophilic matrix tablets [13]. Moreover, when granulation in the wet is used, the nature of the binder neither seems to be really of importance [14]. The results of granulation of a hydrophilic sustained release tablet of verapamil hydrochloride showed that the granulation concerning factors such as granulation fluid volume, mixer speed, mixing time and the use of a wet screening stage had no influence on release rate from the finished tablets. Although these granulation variables showed to be factors affecting mean granule size and with this the flow properties and compressibility of the product [15].
Holgado et al. [9], considered that among factors affecting both drug dissolution in vitro and bioavailability in vivo, could be included the granulation or wetting of the formula components and the variables associated with them. In this case, the release kinetic of carteolol hydrochloride from Eudragit RS matrix tablets was studied using two wetting liquids, Eudragit L 12.5 % and isopropanol-acetone mixture (6:4). The results showing that wetting with the isopropanol-acetone mixture produced the formation of a more compacted matrix structure allowing a slower drug release.
The influence of binding solvents on indomethacin release from hydroxypropyl methylcellulose-lactose tablets was studied at five levels. Within these five levels, four different combinations of water and ethanol were compared with matrix tablets prepared by direct compression [16]. All formulations prepared by direct compression were observed to dissolve less indomethacin, approximately 20 % after 2 h, than any other formulations prepared by wet granulation, which dissolved 20 % to 40 % indomethacin after 2 h. Wet granulation showed a trend to higher dissolution rates with higher water content in the alcohol-water mixture employed as granulation liquid. This was attributed to changes on the degree of swelling and the gel forming ability of HPMC due to the presence of solvent during the preparation of the tablets.
In the present study it was examined the effect of different mixing methods, mortar and pestle mixing, tumbling mixing and wet granulation on the release mechanism and release rate of pelanserin from HPMC-matrix tablets added of different quantities of a water soluble excipient, citric acid.
Pelanserin is an antihypertensive drug possessing several mechanisms of action and effective in reducing blood pressure when administered orally [17, 18]. The sustained release of this experimental drug from matrix tablets obtained by granulation and made of hydroxypropyl methylcellulose (HPMC) and citric acid, previously reported [19], was taken as a reference for the present study.
Materials and methods
Materials. The pharmaceutical excipients hydroxypropyl methylcellulose F4MP (Colorcon), anhydrous citric acid USP (Fisher Scientific Co.) and the experimental drug pelanserin hydrochloride (CINVESTAV-IPN, Mexico) were used as received.
Methods
Matrix preparation. Hydroxypropyl methylcellulose was used to produce matrices containing 20 mg pelanserin hydrochloride loading in different proportions and ratios of HPMC / citric acid mixtures. The powders (10 g) were processed by:
Granulation. Mixing was performed in a twin shell blender with a capacity of 500 mL, constructed with an internal bar, during 20 min at 18 rpm. Thereafter, the mixture was manually granulated, spraying water and kneading 10 min. The wet mass passed finally through a number 14 sieve. The granules were dried at 40° C to get moisture not greater than 4 %, determined by the Karl Fisher method (Aquatest 10).
Mortar and pestle mixing. 10 g of the powder was gently mixed during 10 min with a mortar and pestle.
Tumbling mixing. 10 g of the powder was tumbling mixed in the above-mentioned twin shell blender at 18 rpm during 30 min.
The mixtures were prepared to contain 20 mg pelanserin hydrochloride, 100 mg or 200 mg HPMC and 20, 40, 60, 80, 100 and 120 mg citric acid. Tablets were obtained by compression of powders or granules during 10 sec in a hydraulic press with 8-mm flat-faced punch and die, at a compaction pressure of 55 MPa.
Dissolution methodology. Dissolution studies were carried out at 37° C and 50 rpm, with the USP 23 dissolution apparatus II (paddle method) (Hansen Research) in 900 mL dissolution medium. As usually, considering the pH change from stomach to intestines, for the first 3 h the dissolution medium was HCl 0.1N and then, for the following 5 h, the pH of the medium was adjusted to pH 7.4 by adding 5.0 g of NaOH and 6.12 g of potassium phosphate dissolved in 40 mL water. This new volume was considered to calculate the percentage of pelanserin dissolved.
Samples (3.0 mL) were withdrawn at predetermined time intervals, membrane filtered (φ = 0.45 µm) and analyzed spectrophotometrically at a wavelength of 218 nm (Beckman DU-650 spectrophotometer). Dissolution medium (3.0 mL) was added to maintain a constant volume.
Results and discussion
Pelanserin release from HPMC matrices containing citric acid. The release data from swellable systems like those made of HPMC can be analyzed according to the power law expression shown in equation 1 [16], when delay in release or lag time do not exist:

The terms in this equation are as follows: Mt, the amount of drug released at time t; Minf, the total drug released over a long time period; k, the kinetics constant, also called release constant, and n, the exponent indicating the mechanism of drug release. The value of n ranges from 0.5, (t1/2) dependence generally referred to as Fickian release, to 1.0, representing the case II transport which is purely relaxation controlled. The values in between indicate an anomalous behavior corresponding to coupled diffusion / relaxation mechanism controlling the release process. Although some other theoretical models can be used to describe the dissolution process from matrix tablets, like that of Higuchi or the square root of time, the above-mentioned power law expression is specially convenient to assign a release mechanism to every drug-releasing matrix and to correlate this mechanism with a given release constant. This semi-empirical equation has been used to describe drug release from matrices with different geometries and to describe dissolution data up to 60 %, however it has also been used to describe dissolution up to 90 % of the matrix drug content.
As established in a previous study [19], pelanserin release can be better described applying equation 1 separately for each individual dissolution medium, HCl 0.1 N and phosphate buffer pH 7.4. This can be seen in Fig. 1 for matrices containing 100 mg HPMC per tablet and obtained through mortar and pestle mixing. The points are experimental with their corresponding standard deviation and the lines are those calculated by regression. The correlation coefficients for most of the data were > 0.99.
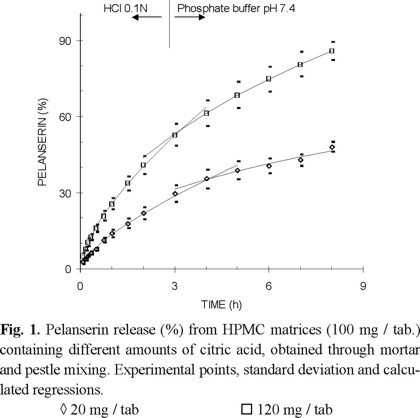
Significance of the mixing method on the release or kinetics constant. Citric acid loading in the range from 20 to 120 mg / tablet, while keeping the drug and polymer content constant modified the release process. Matrices containing 100 mg HPMC / tablet and obtained through tumbling mixing showed that the increase of the citric acid content decreased the ability to sustain drug release. Considering dissolution in HCl 0.1N, the kinetics constant (k) increased from 0.139 to 0.235 when the citric acid content increased from 20 to 120 mg / tablet (Fig. 2). There is a linear relationship between the cube of the citric acid content and the release or kinetics constant [7, 19].
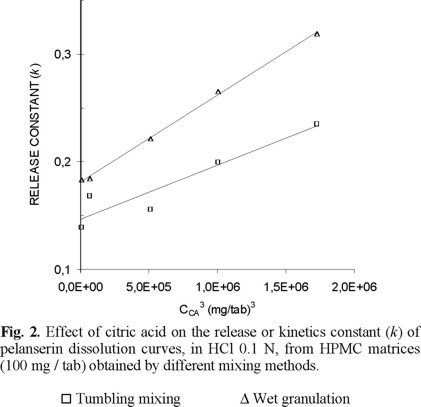
The change in the mixing method from that with mortar and pestle to granulation and tumbling mixing showed a similar relationship between the release constants and the citric acid content (CCA), although the particular values are different.
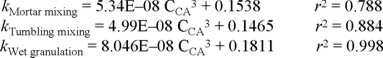
Fig. 2 shows this relationship. The points are experimental and the lines are the calculated regressions. Data corresponding to mortar and pestle mixing were omitted here and in the next figures because of its similarity to tumbling mixing data.
The faster dissolution of tablets obtained by granulation may be attributed to a loosening of the matrix structure generated by HPMC particles surrounded with citric acid. Citric acid is a high soluble material; its granulation with water will dissolve it in a variable degree, depending on the procedure. During granulation, this dissolved citric acid is distributed on the surface of the non-dissolving particles (HPMC). After drying, the dissolved citric acid remains as a solid layer deposited around HPMC. In this way, citric acid acts loosening the matrix structure through an increased porosity created after its dissolution and release and by maintaining separated, in a certain degree, the HPMC particles [7, 19].
In some cases, the effect of processing on the release behavior of hydrophilic matrices containing water-soluble excipients, i.e., citric acid and lactose, seems to be absent. Granulation of verapamil hydrochloride with HPMC, sodium alginate and lactose monohydrate, varying mixing time and speed so as the volume of granulating fluid was reported without effect [15]. No effect was observed because the changes in processing do not affect the distribution of the water-soluble lactose. The lower volume of granulating fluid and the lower mixing time and speed seem to be enough to get a uniform and equal distribution of lactose in the matrix. Further changes increasing the mixing time and speed as well as the volume of the granulating fluid in a magnitude that do not alter the lactose distribution showed no effect on dissolution behavior.
On the other hand, hydrophilic matrices of indomethacin with hydroxypropyl methylcellulose and lactose showed undoubtedly an increased dissolution when obtained after granulation [16]. Matrices mixed by granulation produced about 40 % indomethacin dissolved after two hours while direct compression matrices dissolved only about 20 % indomethacin. Although in this case the increase in dissolution was attributed to changes on the swelling and gel forming capacity of HPMC due to the presence of solvent during granulation, it seems to be more probable due to a change in the lactose distribution. The lactose distribution seems to be also changed by using different proportions of ethanol instead of water. Higher proportions of ethanol reducing lactose dissolution, allowing less lactose distributed on HPMC particles. A discrete distribution of lactose, as particles, allows a more coherent matrix showing lower dissolution. The extreme case is the use of pure ethanol that showed no differences in release behavior compared to direct compression.
In the case of matrix tablets obtained by mortar and tumbling mixing, the citric acid is random distributed as discrete particles. Citric acid acts increasing the porosity of the matrix, after its dissolution and release, but do not prevent the interaction between the HPMC particles that provide a greater coherence to the matrix and a lower dissolution rate. Matrices processed through tumbling mixing and mortar mixing show, comparatively, no significant differences in release rate.
The dissolution behavior of the above-mentioned matrices in phosphate buffer 7.4, after 3 h dissolution in HCl 0.1N, was similar to that observed before. Fig. 3 shows a linear relationship between the release constant (k) and the cube of the citric acid content. Matrices obtained through granulation dissolve faster than those obtained by mortar mixing and tumbling mixing.
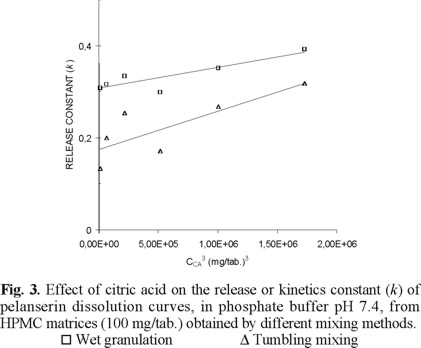
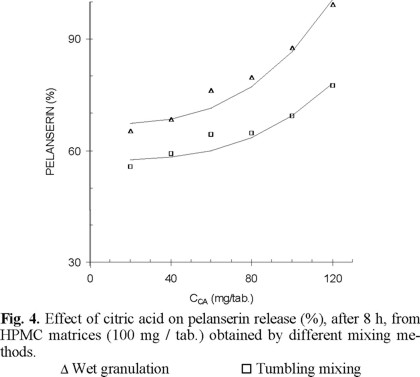
Considering the entire dissolution process, the percentage pelanserin dissolved after 8 h show a similar behavior as that of the release constant against the citric acid content (Fig. 4). After 8 h dissolution, matrices obtained by granulation show about 15-20 % more pelanserin dissolved than matrices obtained by mortar and tumbling mixing. Once again, mortar mixing and tumbling mixing show no significant differences in dissolution. The lines in Fig. 4 are the calculated regressions from the relationship between the percentage pelanserin dissolved against the cube of the citric acid content.
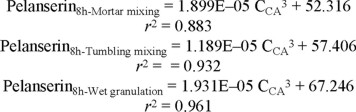
The increase of the polymer content from 100 mg HPMC / tablet to 200 mg HPMC / tablet maintained a greater dissolution rate of matrices obtained through wet granulation, compared to those obtained by mortar and tumbling mixing. However, the release constants showed significant smaller values. The about 30 % decreased values of the release constants obtained from matrices with 200 mg HPMC / tablet made less noticeable the effect of the citric acid content. This and the batch-to-batch variability permitted to simplify the relationship between the release constants and the citric acid content from a cubic to a linear one.
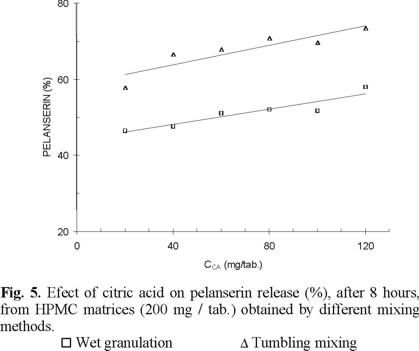
The use of matrices with higher polymer content producing slower release rates gave emphasis to the differences in pelanserin dissolved after 8 h. Matrices with 200 mg HPMC / tablet, obtained by wet granulation, show greater amounts of pelanserin dissolved than those obtained by mortar mixing and tumbling mixing (Fig. 5).
Significance of the mixing method on the release mechanism. To assign the mechanism, it is considered that as the swelling process and the drug elution proceed the gel layer gradually becomes thicker and therefore the drug concentration gradient along the diffusional pathlength is decreased. The gradually decreased drug concentration gradient results in progressively slower drug release rates that in certain proportion can be compensated with increased porosities of the gel layer; these release profiles correspond with n values between 0.5 and 1.0. The continuous polymer hydration during the matrix swelling process will decrease the matrix polymer concentration to a critical value called "disentanglement concentration", which results in gradually increased polymer release rates or erosion of the gel layer. When this erosion is sufficiently high, these release profiles correspond with n values greater than 1.0 [20].
Although the release mechanism can be explained as above-mentioned, drug release from hydrophilic matrices is a multifaceted process where many factors have some influence. It is difficult to separate the effect of one factor from other factors. The assignation of a predominant mechanism is considered here to involve more than a simple process. For a given formula, it is considered that polymer hydration and relaxation constitutes the main variable of the drug release process.
The slopes or n values calculated for dissolution profiles of HPMC matrices (100 mg/tablet), obtained by different mixing methods and dissolving in HCl 0.1N, indicate a mechanism ranging from diffusion to anomalous transport; n values in a range between 0.5 and 0.8 (Fig. 6).
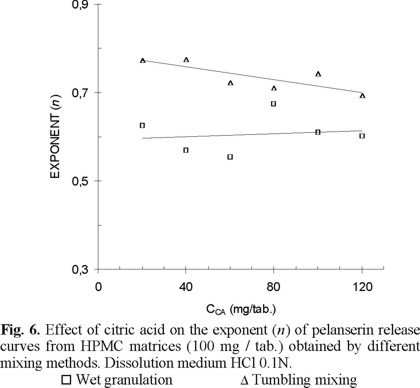
The anomalous transport showed by pelanserin from HPMC matrices obtained by tumbling mixing (nav = 0.605) is nearer drug diffusion than that of matrices obtained by mortar mixing (nav = 0.658) and wet granulation (nav = 0.736). The drug transport in all cases depends upon the influence of both parameters, diffusion through the water filled pores of the matrix and an extra continuous matrix swelling (relaxation / erosion) due to the penetrating fluid.
Mechanistically, matrices obtained by mortar mixing and tumbling mixing show a drug release less dependent on polymer relaxation and nearer to diffusion while matrices obtained by granulation show an equilibrated dependence on drug diffusion and polymer relaxation. These results can be attributed to differences in coherence of the matrix. As explained before, matrices obtained by wet granulation are expected to be less coherent because of the citric acid distribution around the HPMC particles and in this way, allowing a faster absorption of water and less resistance to relaxation. Matrices with discrete citric acid particles are expected to be more coherent, withstanding more the influence of the penetrating fluid to produce polymer relaxation.
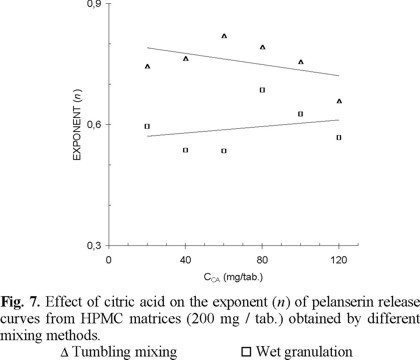
In the case of matrices containing 200 mg HPMC / tablet, the results are similar (Fig. 7). The release mechanism shows the same order of dependence on matrix relaxation than matrices with 100 mg HPMC/tablet. Matrices obtained by granulation showing a drug release with a greater dependence on polymer relaxation and erosion, followed by mortar mixing and finally by tumbling mixing. The exponent n values of matrices containing 200 mg HPMC / tablet show a little greater average than matrices with 100 mg HPMC / tablet. A greater HPMC proportion restricts the solvent penetration and the citric acid release, giving the opportunity for a greater relaxation of the matrix structure.
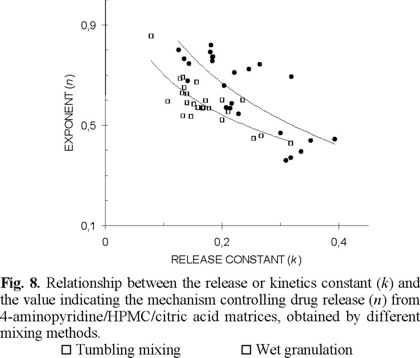
Effect of the mixing method on the relationship release constant/release mechanism. The combined results obtained from dissolution in two different media, six different citric acid proportions, two different HPMC proportions and two different mixing procedures are summarized in Fig. 8. As above-mentioned, this figure does not show the results corresponding to mortar mixing, to allow a better comparison of mixing in the dry to wet granulation. The logarithmic relationship between the exponent n and the corresponding release constant is similar for tumbling mixing and mortar mixing while that obtained for wet granulation is different. There is a trend to higher release constants and higher values of the exponent indicating the release mechanism in matrices obtained by granulation, compared to those obtained by tumbling mixing and mortar mixing. This effect is considered due to differences in the citric acid distribution after processing, as above explained. The equations describing this relationship are as follows:
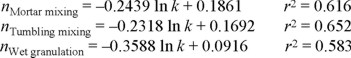
Conclusions
Addition of citric acid to matrices with 100 mg HPMC / tablet produce increases of dissolution rate according to the cube of the citric acid content. The increase of polymer content from 100 mg HPMC / tab, to 200 mg HPMC / tablet reduce significantly the release constants and the citric acid effect, changing the relationship between the release constants and the citric acid content from a cubic to a linear one. The effect of citric acid is attributed to an increased porosity in the matrix after dissolution and release of citric acid. Greater proportions of citric acid produce greater porosities and greater dissolution rates.
Mixing through wet granulation produces matrices with greater drug release constants than those obtained by mortar and tumbling mixing. This is attributed to a change in the citric acid distribution. Tumbling mixing and mortar mixing distribute citric acid as discrete particles that increase the matrix porosity after a rapid dissolution, while wet granulation distribute citric acid not only as discrete particles but as a deposition layer around HPMC particles, decreasing the coherence of the matrix and increasing its porosity.
References
1. Efentakis, M.; Vlachou, M.; Choulis, N. H. Drug Dev. Ind. Pharm. 1997, 23, 107-112. [ Links ]
2. Martini, A.; Montagno, R.; Cappuccinello, R.; Artico, R.; Muggetti, L.; De Ponti, R. Pharm. Sci. 1995, 1, 555-558. [ Links ]
3. Campos-Aldrete, M. E.; Villafuerte-Robles, L. Rev. Mex. C. Farm. 1991, 22, 83-93. [ Links ]
4. Campos-Aldrete, M. E., Villafuerte-Robles, L. Eur. J. Pharm. Biopharm. 1997, 43, 173-178. [ Links ]
5. Mitchell, K.; Ford, J. L.; Armstrong, D. J.; Elliot, P. N. C.; Hogan, J. E; Rostron C. Int. J. Pharm. 1993, 100, 175-179. [ Links ]
6. Kim, H.; Fassihi, R. J. Pharm. Sci. 1997, 86, 323-328. [ Links ]
7. Espinoza-Ramos, R.; Villafuerte-Robles, L. Pharm. Acta Helv. 1999, 74, 65-71. [ Links ]
8. Lapidus, H.; Lordi, N. J. J. Pharm. Sci. 1968, 57, 1292-1301. [ Links ]
9. Holgado, M. A.; Caraballo, I.; Álvarez-Fuentes, J.; Fernández-Hervás, M. J.; Fernández-Arévalo, M.; Rabasco, A. M. Int. J. Pharm. 1995, 118, 151-160. [ Links ]
10. Veiga, F.; Salsa, T.; Pina, M. E. Drug Dev. Ind. Pharm. 1997, 20, 2519-2526. [ Links ]
11. Traconis, N., Rodríguez, R., Campos, M. E., Villafuerte, L. Pharm. Acta Helv. 1997, 72, 131-138. [ Links ]
12. Juárez, H.; Rico, G.; Villafuerte, L. Int. J. Pharm. 2001, 216, 115-125. [ Links ]
13. Alderman, D. A. Int. J. Pharm. 1984, 5 (3), 1-9. [ Links ]
14. Vázquez, M. J.; Pérez-Marcos, B.; Gómez-Amoza, J. L.; Martínez-Pacheco, R.; Souto, C.; Concheiro, A. Drug Dev. Ind. Pharm. 1992, 18, 1355-1375. [ Links ]
15. Timmins, P.; Delargy, M. A.; Howard, J. R.; Rowlands, E. A. Drug Dev. Ind. Pharm. 1991, 17, 531-550. [ Links ]
16. Mandal, T. K. Drug Dev. Ind. Pharm. 1995, 21, 1389-1397. [ Links ]
17. Flores-Murrieta, F. J.; Castañeda-Hernández, G.; Hong, E. Arzneim.-Forsch. / Drug Res. 1992, 41, 9, 1105-1108. [ Links ]
18. Flores-Murrieta, F. J.; Herrera, J. E.; Castañeda-Hernández, G.; Hong, E. Proc. West. Pharmacol. Soc. 1992, 35, 113-116. [ Links ]
19. Espinoza-Ramos, R.; Hong-Chong, E.; Villafuerte-Robles, L. Int. J. Pharm. 2000, 201, 165-173. [ Links ]
20. Sung, K. C.; Nixon, P. R.; Skoug, J. W.; Ju, T. R.; Gao, P.; Topp, E. M.; Patel, M. V. Int. J. Pharm. 1996, 142, 53-60. [ Links ]














