Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Sociedad Química de México
versión impresa ISSN 0583-7693
Rev. Soc. Quím. Méx vol.46 no.3 Ciudad de México jul./sep. 2002
Investigación
Triterpenes, Phenols, and Other Constituents from the leaves of Ochroma pyramidale (Balsa Wood, Bombacaceae). Preferred Conformations of 8-C-β-D-Glucopyranosyl-apigenin (vitexin)
Erika Vázquez,1 Esteban M. Martínez,2 Juan Antonio Cogordán,1 and Guillermo Delgado1*
1 Instituto de Química.
2 Instituto de Biología (Herbario Nacional) de la Universidad Nacional Autónoma de México. Circuito Exterior, Ciudad Universitaria. Coyoacán 04510. México, D. F. Telephone: (52)-(55)-5622-4446. E-mail: delgado@servidor.unam.mx
Recibido el 22 de julio del 2002.
Aceptado el 24 de septiembre del 2002.
Abstract
Lupeol, oleanolic acid, stigmasterol, β-sitosterol, β-sitosteryl-β-D-glucopyranoside, catechin, epicatechin, and 8-C-β-D-glucopyranosylapigenin (vitexin) were isolated from the acetonic extract of the leaves of Ochroma pyramidale (balsa wood, Bombacaceae), a tree noted by its exceedingly light wood. 1H and 13C NMR of 8-C-β-D-glucopyranosyl-apigenin (vitexin) at room temperature exhibited doubling of some signals, suggesting the presence of atropisomers. 1H NMR spectra at 70 °C showed one set of signals, confirming the presence of rotational isomers at room temperature. Molecular modelling using AM1 methods, established the two conformations of minimum energy for vitexin, which correspond to the -ac (ω = -94 °) and +ac (ω = + 96 °) arrangement along the C(2")-C(1")-C(8)-C(7) bonds.
Keywords: Ochroma pyramidale, balsa wood, Bombacaceae, triterpenes, phenols, sterols, conformational analysis, mollecular modeling, vitexin.
Resumen
Del extracto acetónico de las partes aéreas de Ochroma pyramidale (madera balsa, Bombacaceae), un árbol que se caracteriza por la ligereza de su madera, fueron aislados lupeol, ácido oleanólico, estigmasterol, β-sitosterol, β-D-glucopiranósido de β-sitosterilo, catequina, epicatequina, and 8-C-β-D-glucopiranosil apigenina (vitexina). Algunas señales en los espectros de RMN de 1H y 13C de esta última substancia mostraron duplicidad a temperatura ambiente, sugiriendo la presencia de atropisómeros. El espectro de RMN 1H a 70 °C mostró solo un conjunto de señales, confirmando la presencia de rotámeros a temperatura ambiente. El modelaje molecular usando métodos AM1 de la vitexina estableció las dos conformaciones de mínima energía de la molécula, las cuales corresponden a los arreglos -ac (ω = -94°) and +ac (ω = + 96°) de los enlaces C(2")-C(1")-C(8)-C(7).
Palabras clave: Ochroma pyramidale, madera balsa, Bombacaceae, triterpenos, fenoles, esteroles, análisis conformacional, modelaje molecular, vitexina.
Dedicated to Dr. Barbarín Arreguín Lozano.
Ochroma pyramidale (syn: O. lagopus [1]; common name: balsa wood) is a tree of the Bombacaceae family [2], growing in tropical areas of Meso-, Central- and South America and noted by its very light wood [3]. Balsa wood has a very low density (0.110-0.200 g / cm3) [4] and because of its buoyancy, it is used for making floats. Its resilency makes it an excellent shock-absorbing material and because of its lightness and insulating properties, it is a valuable construction material. Balsa seed fibre is used commercially as plant eiderdown [5]. From the ethanolic extract of the wood were previously isolated fatty acids, sterols, coumarins and lignanes [6], however, the leaves, which are considered as a waste material by the forest industry, have not been previously analyzed. This paper describes the secondary metabolites isolated from the leaves of balsa tree and the preferred conformations of 8-C-β-D-glucopyranosylapigenin (vitexin), determined by molecular modelling.
The leaves of O. pyramidale were extracted at room temperature with acetone, and the acetonic extract was purified by silica gel column chromatography and preparative TLC on silica gel, to afford eight compounds. Lupeol [7], oleanolic acid [8], stigmasterol [9], β-sitosterol [9], and β-sitosteryl-β-D-glucopyranoside [7] were identified by comparing their spectroscopic data with those previously reported.
From the polar fractions was isolated a solid which was a mixture of two compounds (1a + 2a), according to its 1H NMR data. Acetylation of this mixture afforded the peracetylated compounds which were separated by preparative TLC. Peracetylcatechin (1b) and peracetyl-epi-catechin (2b) were identified as the less polar and the more polar compounds, respectively, and their 1H and 13C NMR assignments are listed in the experimental. Therefore, catechin (1a) and epi-catechin (2a) [10] are the natural compounds from the leaves of balsa wood.
The positive FABMS of the additional compound showed an [M+ + 1] peak corresponding to the formula C21H20O10, in agreement with the number of signals observed in the 13C NMR spectrum and the number of protons registered in the 1H NMR spectrum. Its IR spectrum showed absorption bands at 3380-3251 and 1655 cm-1 for hydroxyl and α,β,γ,δ-unsaturated carbonyl groups, respectively. Its 13C NMR spectrum showed 19 resonances, sorted by DEPT experiments in one methylene, nine methines and nine quaternary carbons. The presence of the flavonoid skeleton was evident by the typical AA'BB' proton doublets centered at δH 8.02 and 6.88 (2H each), and the singlets at δH 6.77 and 6.27 (1H each) for the H-3 and one A-ring proton, respectively, in the 1H NMR spectrum. These assignments were confirmed by the HMQC experiment, which showed the corresponding crosspeaks at δC 128.90, 115.73, 102.41 and 98.06 for C(2' + 6'), C(3' + 5'), C(3) and the corresponding carbon of the A ring, respectively. The second hydroxyl could be located at C(7), as in the apigenin derivatives. The presence of an hexose was evident by the presence of six signals in the 13C NMR spectrum between 60 and 82 ppm which correlated in the HMQC with the signals between 3.00 and 4.70 ppm in the 1H NMR spectrum. COSY and TOCSY experiments established the connectivity for the hexopyranose which was determined as a β-D-glucopyranose by the all trans-diaxial coupling pattern within the pyranoid system, yielding only large coupling constants (10 Hz) for H-1' through H-5'. The presence of the C(8)-C(1") bond (C-glycosylflavonoid, instead of O-glycosylflavonoid) was determined by the upfield shift of H-1" (δH 4.67) and by the observed crosspeaks between H-1" and C-8 (δC 104.55), C-8a (δC 162.5) and C-7 (δC 155.99) in the HMBC spectrum. Therefore, this substance was established as 8-C-β-D-glucopyranosylapigenin (vitexin). Direct comparison of the physical properties with those published [11], confirmed its identity.
Several signals in the 1H and 13C NMR spectra of 3 exhibited some doubling or broadening at room temperature, as previously noted for some C-glycosides of flavonoids [12, 13], due to the presence of atropisomers. When the 1H NMR spectra of 3 was ran at 70 °C, the signals sharpened, confirming the interconvertibility of the rotational isomers. Conformers A-D in Fig. 1 represent four possible rotamers derived from rotation (90°) along the C(1")-C(8) sigma bond of vitexin (3). Considering the C(2")-C(1")-C(8)-C(7) torsion angles (ω), these conformations could be described as + synclinal (ω = 60°, A), Ψ-antiperiplanar (ω = 150°, B), -anticlinal (ω = -120°, C), and Ψ-synperiplanar (ω = -30°, D).

In order to determine the preferred conformations of 3, molecular structure calculations at semiempirical level were carried out. AM1 parametrization was used, and this method has proved to provide good molecular conformations for organic molecules [14, 15]. For the calculations the AM1 implementation in the suit of programs Gaussian 98 was used [16, 17].
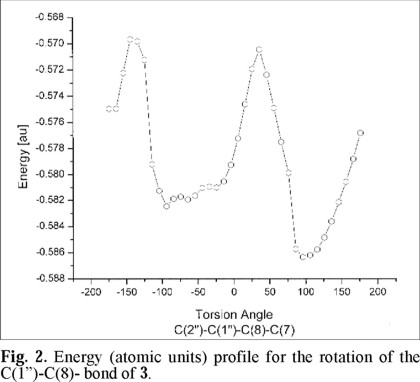
Since the β-D-glucopyranose and the C-ring of the flavone are molecular fragments able to rotate in solution, around the C(1")-C(8) and C(2)-C(1') bonds, respectively, any attempt to find a low energy conformation should consider the rotations along these two sigma bonds. The symmetry observed for the C-ring suggests small deviations of the dihedral angle of this ring with respect to the plane of the A- and B- rings of the flavone nucleus. Based on this last observation, geometry optimization was applied to a set of values varying the dihedral angle for the β-D-glucopyranose fragment and the A/B rings of the flavonoid along the C(1")-C(8) bond. In this dynamical potential energy surface scan, the torsion angles C(2")-C(1")-C(8)-C(7) are kept fixed in each run and all the other degrees of freedom are optimized. In Fig. 2 the results of the computations are displayed. In these calculations the torsion angle (ω1) C(2")-C(1")-C(8)-C(7) was varied every 10 degrees, starting from an optimized structure. It may be observed that there are two possible minima: one at -94 ° (-ac conformation along the indicated bonds, see Fig. 4A), with an energy of -0.582 atomic units (au), and the second at +96 ° (+ac conformation, see figure 4B), with an energy of -0.586 au. It may be noted that around these minima there are other values of the torsion angle which are energetically accessible to the structure, indicating that the molecule may be in any of these conformations. The difference between the two minima was estimated in 3.89 × 10-3 au (2.4 kcal mol-1) and the energy barrier for interconversion may be calculated in 0.120 au (7.5 kcal mol-1). This barrier is too low for separation to be possible [18].
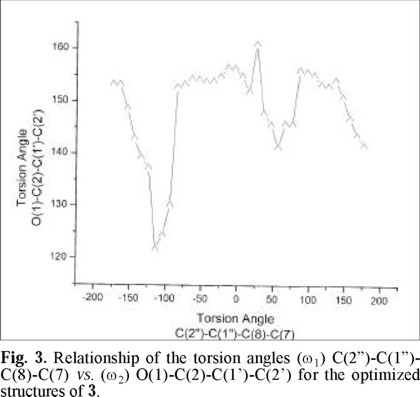
In Fig. 3 the graph of the torsion angle (ω2) O(1)-C(2)-C(1')-C(2') as a function of the torsion angle (ω1) C(2")-C(1")-C(8)-C(7), is depicted. The values of ω1 = -94° and ω2 = 130° correspond to the first minimum and the values ω1 = + 96° and ω2 = 156° correspond to the lowest minimum. It may be noticed that the C-ring tends to be tilted with respect to the plane of the AB rings of the flavone in all the optimized structures, and this tendency for non-planarity is imposed by steric demands.

In Fig. 4 the molecular conformations (A and B) corresponding to each minimum in energy are shown. 4A corresponds to the first energy minimum, and there are similar minima as the torsional angle (ω1) C(2"')-C(1"')-C(8)-C(7) increases (from -94° to -25°, -ac conformation, see Fig. 2). We may notice that the C-ring is tilted (ω2 = 130°) with respect to the A / B rings in this conformation. Figure 4B corresponds to the conformation of lowest energy (+ ac conformation) and in this case, the C-ring is less tilted (ω2 = 156°) than in 4A. The energy minima in conformation 4B may be consequence of Van der Wals and even to a weak hydrogen bond interactions between atoms in the β-D-glucopyranose and the C-ring fragment. This interaction is around 7 kcal-mol-1.
Some bioactivities of the substances isolated from the leaves of balsa wood are known. The anti-inflammatory properties of lupeol have been studied [19]. Lupeol and oleanolic acid have been recently evaluated as cytotoxic agents [20], and this last substance has been identified as an anti-HIV constituent from several plants [21]. Catechin (1a) was effective for the treatment of osteoarthritis [22], and some plant phenols have been tested for the treatment of burns [23]. Vitexin (3) has been evaluated on some receptors using ligand-binding bioassays [24]. Therefore, the aerial parts of the balsa wood trees, considered a waste for the forest industry, contain several bioactive substances. Additional studies will be needed to determine the ontogenic and seasonal effects on the secondary metabolites content of the leaves, considering the harvest of the tree.
Experimental section
General experimental procedures. Column chromatographies were performed on Merck silica gel 60, 0.040-0.063 mm, 230-400 mesh. Spots were detected on TLC by heating after spraying with 1% (NH4)4Ce(SO4)4 in 2N H2SO4. MS were obtained in a JEOL JMX-SX102A spectrometer. 1H and 13C NMR were recorded on a Varian 500 Unity plus. Chemical shifts are reported in δ (ppm). Melting points are uncorrected.
Plant Material. Leaves of Ochroma pyramidale (Cav. ex Lam.) Urban (Bombacaceae) was collected in Ocosingo, Chiapas, México. A voucher specimen (M-17546) was deposited in the National Herbarium (MEXU), Instituto de Biología de la Universidad Nacional Autónoma de México. The plant was collected and identified by E. M. M.
Extraction and Isolation. Powdered, dried leaves (1.2 kg) were extracted with acetone three times at room temperature for 48 h each time. The extracts were combined and evaporated at reduced pressure to give 22 g of a syrup. Part of the extract (17 g) was adsorved onto silica gel (20 g) and chromatographed on a silica gel column (160 g) using VCC [25], and eluting with CH2Cl2 and CH2Cl2-acetone gradient. The eluted fractions were evaluated by TLC to give ten main fractions (A-J). Fraction A was constituted by fatty material and glicerides. Rechromatography of the fractions B and C (2.3 g) afforded stigmasterol and β-sitosterol (320 mg as a 1:1 mixture). Fraction D (100 mg) was rechromatographed on a silica gel column eluting with CH2Cl2-acetone, to obtain lupeol (28 mg). Rechromatography of the fractions E-G (1.8 g) on a silica gel column eluting with CH2Cl2-acetone afforded 85 mg of oleanolic acid. β-Sitosteryl-β-D-glucopyranoside (52 mg) was obtained by rechromatography of the fraction H (700 mg). From the column rechromatography of the fraction I (1.1 g) was obtained a powder (90 mg, 1a + 2a) which was acetylated following the usual procedure to obtain a residue (110 mg) which was purified by preparative TLC (eluting with CH2Cl2-acetone 94:6) to afford peracetyl-catechin (1b, 46 mg) and peracetyl-epi-catechin (2b, 40 mg). Finally, fraction J (2 g) was chromatographed on a silica gel column eluted with CH2Cl2-acetone gradient to obtain a solid which was recrystallized several times from MeOH, to obtain 14 mg of 8-C-β-D-glucopyranosylapigenin (vitexin, 3).
Pentaacetyl-catechin (1b): pale yellow solid. mp: 130-132 °C; Rf : 0.61 (CH2Cl2-acetone, 96:4); [α]22D + 28 (c 1.28 mg / mL, CHCl3), 1H NMR (500 MHz, CDCl3): δ 7.25 (1H, ddd, J = 8.5, 2.0, 0.5, H-6'), 7.19 (1H, d, J = 8.5, H-5'), 7.17 (1H, d, J = 2.0, H-2'), 6.66 (1H, d, J = 2.0, H-6), 6.60 (1H, d, J = 2.0, H-8), 5.25 (1H, ddd, J = 6.5, 6.5, 5.0, H-3), 5.14 (1H, dd, J = 6.5, 0.5, H-2), 2.87 (1H, dd, J = 17.0, 5.0, H-4a), 2.66 (1H, dd, J = 17.0, 6.5, H-4b), 2.28 (9H, s, CH3CO-), 2.27 (3H, s, CH3CO), 2.00 (3H, s, CH3CO); 13C NMR (125 MHz, CDCl3): δ 149.89 (C-7), 149.46 (C-5), 142.14 (C-1'), 136.15 (C-3', C-4'), 124.40 (C-6'), 123.70 (C-5'), 121.79 (C-2'), 108.76 (C-6), 107.68 (C-8), 77.67 (C-2), 68.39 (C-3), 23.91 (C-4).
Pentaacetyl-epi-catechin (2b): pale yellow solid, mp: 150-152 °C; Rf: 0.70 (CH2Cl2-acetone, 96:4); [α]22D - 17.4 (c 1.38 mg / mL, CHCl3), 1H NMR (500 MHz, CDCl3): δ 7.35 (1H, d, J = 2.0, H-2'), 7.27 (1H, dd, J = 8.0, 2.0, H-6'), 7.21 (1H, d, J = 8.0, H-5'), 6.67 (1H, d, J = 2.0, H-8 or H-6), 6.57 (1H, d, J = 2.0, H-6 or H-8), 5.39 (1H, m, H-3), 5.11 (1H, brs, H-2), 2.98 (1H, dd, J = 18.0, 4.5, H-4a), 2.89 (1H, dd, J = 18.0, 1.5, H-4b), 2.30 (3H, s, CH3CO-), 2.29 (6H, s, CH3CO), 2.28 (3H, s, CH3CO), 1.92 (3H, s, CH3CO).
8-C-β-D-glucopyranosylapigenin (vitexin, 3): pale yellow solid, mp: 262-264 °C (dec), Rf: 0.79 (CH2Cl2-MeOH, 4:1); [α]22D - 7.7 (c 1.30 mg / mL, Py-d5); IR (KBr) νmax 3379, 3251, 2912, 1655, 1613, 1569, 1507, 1362, 1294, 1178, 1092 cm-1; 1H NMR (500 MHz, DMSO): δ 13.16 (1H, brs, 5-OH), 10.82 (brs, 7-OH), 10.34 (1H, brs, 4'OH), 8.01 (2H, d, J = 9.0, H-2', H-6'), 6.88 (2H, d, J = 9.0, H-3', H-5'), 6.78 (1H, s, H-3), 6.27 (1H, s, H-6), 4.98 (1H, d, J = 5.5, OH), 4.95 (1H, d, J = 3.6, OH), 4.68 (1H, d, J = 10.0, H-1"), 4.58 (1H, brs, OH), 3.83 (1H, dd, J = 10.0, 10.0 Hz), 3.75 (1H, dd, J = 12, 6.5, H-6"a), 3.51 (1H, dd, J = 12.0, 6.0, H-6"b), 3.36 (1H, d, J = 10.0, H-4"), 3.30 (1H, d, J = 10.0, H-3"), 3.24 (1H, m, H-5"); 13C NMR (125 MHz, CDCl3): δ 182.05 (C-4), 163.91 (C-2), 162.50 (C-9), 161.04 (C-4'), 160.37 (C-5), 155.99 (C-7), 128.90 (C-2', C-6'), 121.60 (C-1'), 115.73 (C-3', C-5'), 104.55 (C-8), 103.99 (C-10), 102.41 (C-3), 98.06 (C-6), 81.75 (C-5"), 78.58 (C-3"), 73.32 (C-1"), 70.74 (C-2"), 70.49 (C-4"), 61.24 (C-6); FABMS (NOBA+) m/z (rel. int.) 433 (M++1)(9), 329 (4), 307 (25), 289 (15), 257 (3), 242 (4), 226 (2), 176 (9), 154 (100), 136 (69), 107 (20), 89 (17), 77 (15).
Acknowledgments
The authors thank Rocío Patiño, María Isabel Chávez, Beatriz Quiroz, Luis Velasco and Javier Pérez-Flores, of the Instituto de Química de la Universidad Nacional Autónoma de México for technical assistance. Financial support from Consejo Nacional de Ciencia y Tecnología (3699P-E9608) is gratefully acknowledged.
References
1. Pierce, J. H. Trop. Woods, Yale Univ. Sch. For. 1942, 69, 1-2. [ Links ]
2. Miranda, F. La Vegetación de Chiapas. Ed. del Gobierno del Estado. Tuxtla Gutiérrez, Chiapas. México. 1952. [ Links ]
3. Fletcher, M. I. Econ. Bot. 1951, 5, 107-125. [ Links ]
4. Pierce, J. H. Trop. Woods, Yale Univ. Sch. For. 1942, 69, 1-2. For comparison, basswood (Tilia glabra), pine (Pinus palustris) and mahogany (Switenia macrophylla) have density values of 0.398, 0.638 and 0.540 g / cm3, respectively. Physical properties of com-mon woods. http://www.csudh.edu/oliver/pubdomdb.htm [ Links ]
5. Pierce, J. H. J. New York Bot. Gard. 1942, 43, 268-277. [ Links ]
6. Paula, V. F.; Barbosa, L. C.; Howarth, O. W.; Demuner, A. J.; Cass, Q. B.; Vieira, I. J. C. Tetrahedron 1995, 51, 12453-12462. [ Links ]
7. Viqar Uddlin, A.; Attaur-Rahman. Handbook of Natural Products Data. Pentacyclic Triterpenoids. Vol 2. Elsevier, Amsterdam. 1994. [ Links ]
8. Shao, Ch.-J.; Kasai, R.; Xu, J.-D.; Tanaka, O. Chem. Pharm. Bull. 1989, 37, 42-45. [ Links ]
9. (a) Horibe, I.; Nakai, H.; Sato, T.; Seo, S.; Takeda, K. J. Chem. Soc. Perkin Trans. 1 1989, 1957-1967. [ Links ] (b) Garg, V. K.; Nes, W. R. Phytochemistry 1984, 23, 2925-2929. [ Links ]
10. Thompson, R. S.; Jacques, D.; Haslam, E.; Tanner, R. J. J. Chem. Soc. Perkin 1 1972, 1387-1399. [ Links ]
11. Mabry, T. J.; Markham, K. R.; Thomas, M. B. The Systematic Identification of Flavonoids. p. 282. Springer-Verlag. 1970. [ Links ]
12. Markham, K. R.; Mues, R.; Stoll, M.; Zinsmeister, H. D. Z. Naturforsch. 1987, 42c, 1039-1042. [ Links ]
13. Harborne, J. B., Ed. The Flavonoids. Advances in Research. Ch. 3. Chapman and Hall, 1994. [ Links ]
14. Stewart, J. J. P. Semiempirical Molecular Orbital Methods, in Reviews in Computational Chemistry, Vol.1, Kenny B. Lipkowitz and Donald B. Boyd, Editors. Wiley-VCH. [ Links ]
15. Zerner, M. Z. Semiempirical Molecular Orbital Methods, in Reviews in Computational Chemistry, Vol. 2, Lipkowitz, K. B. and Boyd, D. B. Editors. Wiley-VCH. [ Links ]
16. Gaussian 98, Revision A.7. Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Zakrzewski, V. G.; Montgomery, Jr.J. A.; Stratmann, R. E.; Burant, J. C.; Dapprich, S.; Millam, J. M.; Daniels, A. D.; Kudin, K. N.; Strain, M. C.; Farkas, O.; Tomasi, J.; Barone, V.; Cossi, M.; Cammi, R.; Mennucci, B.; Pomelli, C.; Adamo, C.; Clifford, S.; Ochterski, J.; Petersson, G. A. ; Ayala, P. Y.; Cui, Q.; Morokuma, K.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Cioslowski, J.; Ortiz, J. V.; Baboul, A. G.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Gomperts, R.; Martin, R. L.;. Fox, D. J.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; A. Nanayakkara, A.; González, C.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, W. W.; Andres, J. L.; Gonzalez, C.; Head-Gordon, M.; Replogle, E. S.; Pople, J. A. Gaussian, Inc., Pittsburgh PA, 1998. [ Links ]
17. MOLEKEL 4.1, Flükiger, P.; Lühti, H. G.; Portmann, S.; Weber, J. Swiss Center for Scientific Computing, Manno (Switzerland), 2000-2001. MOLEKEL is a program distributed free of charge. Available from: http://www.scsc.sw/molekel [ Links ]
18. Eliel, E.; Wilen, S. H. Stereochemistry of Organic Compounds. John Wiley & Sons, Inc. 1994. [ Links ]
19. Recio, C. M.; Giner, R. M.; Manez, S.; Rios, J. L. Planta Med. 1995, 61, 182-185. [ Links ]
20. Hata, K.; Hori, K.; Takahashi, S. J. Nat. Prod. 2002, 65, 645-648. [ Links ]
21. Kashiwada, Y.; Wang, H.-K.; Nagao, T.; Kitanaka, S.; Yasuda, I.; Fujioka, T.; Yamagishi, T.; Cosentino, L. M.; Kozuka, M.; Okabe, H.; Ikeshiro, Y.; Hu, Ch.-Q.; Yeh, E.; Lee, K.-H. J. Nat. Prod. 1998, 61, 1090-1095. [ Links ]
22. Niebes, P. J.; Vincze, A. B.; Roba, J. L.; Lambelin, G. E.; Matagne, D. M.; Hanon, E. T.; Franz, M. R. U. S. Patent No. 4,368,847, 1981, May 19. [ Links ]
23. Koganov, M. M.; Dueva, O. V.; Tsorin, B. L. J. Nat. Prod. 1999, 62, 481-483. [ Links ]
24. Hasrat, J. A.; Pieters, L.; Claeys, M.; Vlietinck, A.; De Backer, J.-P.; Vauquelin, G. J. Nat. Prod. 1997, 60, 638-641. [ Links ]
25. Salituro, G. M.; Dufresne, C. Isolation by Low Pressure Column Chromatography. Natural Products Isolation. Cannell, R. J. P. (Ed.) Humana Press Inc. 1998. [ Links ]














