Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de la Sociedad Química de México
Print version ISSN 0583-7693
Rev. Soc. Quím. Méx vol.46 n.3 Ciudad de México Jul./Sep. 2002
Investigación
Crystal and Molecular Structure of 2-Aminoacetophenone-N(3)-dimethylthiosemicarbazone
Amal A. Nassar,a Simón Hernández-Ortega,b Jesús Valdés-Martínez*b and Douglas X. Westc
a Department of Chemistry, El-Menoufia University, Shebin El-Kom, Egypt.
b Instituto de Química, Universidad Nacional Autónoma de México, Cicuito Exterior, Cuidad Universitaria, Coyoacán 04510, México D.F. Tel:+52-55-5622-4514; Fax:+52-55-5616-2217. E-mail: jvaldes@servidor.unam.mx
c Department of Chemistry 351700, University of Washington, Seattle, WA 98195-1700, USA.
Recibido el 4 de abril del 2002.
Aceptado el 6 de septiembre del 2002.
Abstract
Condensation of N(3)-dimethylthiosemicarbazide with 2-aminoacetophenone in anhydrous ethanol produces 2-aminoacetophenone-N(3)-dimethylthiosemicarbazone, 1, which is planar with the S1 and N1 atoms in a syn conformation. The amino group forms a bifurcated intramolecular hydrogen bond with the sulfur and the imine nitrogen, and an intermolecular hydrogen bond with the thione sulfur of a second molecule.
Keywords: Thiosemicarbazones, Crystal structure, X-Ray.
Resumen
La condensación de la N(3)-dimetiltiosemicarbazida con la 2-aminoacetofenona en etanol anhidro produce la 2-aminoacetofenona-N(3)-dimetiltiosemicarbazona, 1, la molécula es plana y los átomos S1 y N1 se encuentran en una conformación syn. El grupo amino forma un enlace de hidrógeno intramolecular bifurcado con el azufre y el nitrógeno de tipo imina, y un enlace de hidrógeno intermolecular con el azufre de una segunda molécula.
Palabras clave: Tiosemicarbazonas, estructura cristalina, Rayos X.
Al Dr. Barbarín Arreguín Lozano
Introduction
Although there has been considerable interest in thiosemicarbazones derived from salicylaldehyde [1] and 2-hydroxyace-tophenone [2], and in particular, those with substitution at the N(3)-position of the thiosemicarbazone moiety [3-5], less attention has been given to the 2-aminobenzaldehyde [6] and 2-aminoacetophenone thiosemicarbazones [7, 8]. The amino group provides additional hydrogen bonding possibilities, a major interest in the structural studies of thiosemicarbazones [9] and thioureas [10]. We previously prepared and characterized representative copper(II) [11], nickel(II) [12] and cobalt(II) [13] complexes of a selection of 2-aminoacetophenone N(3)-substituted thiosemicarbazones, as well as the unsubstituted thiosemicarbazone. Although we have not obtained suitable crystals of any of the complexes, in this paper we present the molecular and crystal structure of the 2-aminoacetophe-none-N(3)-dimethylthiosemicarbazone, 1.
Results and Discussion
Table 1 summarizes the crystal data, collection information and refinement data of 1. Selected bond distances and angles are in Table 2, and Table 3 provides information about the hydrogen-bond geometries. Figure 1 shows the molecular geometry, thermal ellipsoids (30 %), and numbering scheme.


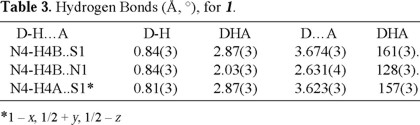

The S1 and N1 atoms of 1 are in a syn conformation with respect to the C8-N2 bond, and the amino group on the aromatic ring forms an intramolecular bifurcated hydrogen bond with S1 and N2, giving the molecule the conformation presented in Fig. 1. The C8-S1 bond distance is consistent with its assignment as a formal double bond, and similar to distances found for the analogous bond in thioureas [10] and other thiosemicarbazones [9]. The C7-N1 bond is formally a double bond. The C2-N4, C8-N3 and C8-N2 bond lengths indicate delocalization [14], and the average bond angles around N2 (119.5°), N3 (120.0°) and N4(119.4°) correspond to an sp2 hybridization. The N1-N2 bond distance is shorter than the observed value (1.401 Å) in single N-N bonds [14]. In agreement with the bonding delocalization observed in the molecule, torsion angles indicate the molecule is planar. All the atoms other than hydrogens lay in essentially the same plane, with C11 (0.277(3) Å) and N4 (0.275(3) Å) showing the largest deviation from the mean plane of the molecule.
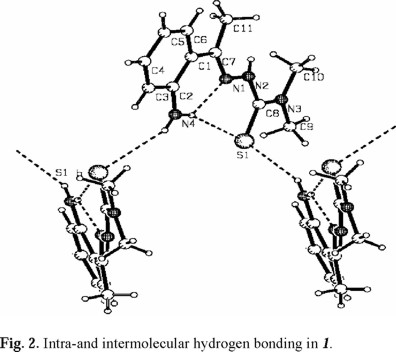
In addition to the intramolecular hydrogen bonds, the amino group is involved in an intermolecular N4-H...S1 (1 - x,1/2 + y, 1/2 - z) hydrogen bond, which generates infinite chains, as shown in Fig. 2. To improve this intermolecular hydrogen bond the NH2 needs to deviate from the mean plane of the molecule, this deviation is reflected in the 13.8° angle observed between the mean plane of the aromatic ring and the mean plane formed by C11-C7-N1-N2-C8-S1-N3. The hydrogen atom on N2 does not participate in hydrogen bonding probably due to the steric hindrance of the C10 and C11 methyl groups.
Experimental
2-aminoacetophenone was purchased from Aldrich and used without further purification. N(3)-dimethylthiosemicarbazide was prepared following the method of Scovill [15]. The thio-semicarbazide was refluxed for ca. 2 h. with 2-aminoacetophenone in ethanol containing a few drops of conc. H2SO4 as reported previously [11].
Crystals of 1 were obtained by slow evaporation of dilute ethanol solutions and mounted in random orientation on glass fibers. Reflections were acquired with a Nicolet P3/F diffractometer. Three standard reflections every 97 reflections were used to monitor the crystal stability. The structure was solved by direct methods, missing atoms were found by difference-Fourier synthesis, and refined on F2 by a full-matrix least-squares procedure using anisotropic displacement parameters using SHELX-97 [16]. Hydrogen atoms attached to nitrogens were found on a difference Fourier map and refined isotropically while hydrogens attached to carbons were located in their calculated positions (C-H, 0.93-0.97 Å), and refined using a riding model. Scattering factors are from the International Tables for Crystallography (1974, Vol IV) [17]. The graphics used are PLATON [18].
Supporting Information Available. CCDC 185211 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk).
References
1. West, D.X.; Salberg, M.M.; Bain, G.A.; Liberta, A.E.; Transition Met. Chem. 1997, 22, 180-184, and refs therein. [ Links ]
2. Dey, K.; Bandyopadhyay, D. Ind J. Chem. 1992, 31A, 34-38. [ Links ]
3. Lu, Z.; White, C., Rheingold, A. L.; Crabtree, R. H. Inorg. Chem. 1993, 32, 3991-3195. [ Links ]
4. West, D.X.; Yang, Y-H.; Klein, T.L.; Goldberg, K.I.; Liberta, A. E.; Valdés-Martínez, J.; Toscano, R. A. Polyhedron 1995, 14, 1681-1693. [ Links ]
5. West, D.X.; Yang, Y-H.; Klein, T.L.; Goldberg, K. I.; Liberta, A.E.; Valdés-Martínez, J.; Hernández-Ortega, S. Polyhedron 1995, 14, 3051-3060. [ Links ]
6. Ablov, A.V.; Rotaru, V.K.; Kiosse, G.A.; Malinovskii, T.I.; Shopron, M.V.; Gerbeleu, V. Dokl. Akad. Nauk SSSR 1973, 208, 353-355. [ Links ]
7. El-Asmy, A.A.; Shaibi, Y. M.; Shedaiwa I. M.; Khattab, M.A. Synth React. Inorg. Metorg Chem. 1988, 18, 331-345. [ Links ]
8. Abu El-Reash, G.M.; Ibrahim, K. M.; Rakha, T. H. Transition Met. Chem., 1989, 14, 209-212. [ Links ]
9. West, D.X.; Bain, G.A.; Butcher, R. J.; Jasinski, J.P.; Li, Y.; Pozdniakiv, R.Y.; Valdés-Martínez, J.; Toscano, R.A.; Hernández-Ortega, S. Polyhedron 1996, 15, 665-674; [ Links ] Valdés-Martínez, J.; Hernández-Ortega, S.; West, D.X.; Stark, A.M.; Bain, G.A. J. Chem Cryst. 1996, 26, 861-864. [ Links ] Valdés-Martínez, J.; Hernández-Ortega, S.; West, D.X.; Ives, J. S.; Bain, G. A.; Z. Krystallogr. 1998, 213, 246-248; [ Links ] Castiñeiras, A.; Bermejo, E.; Ackerman, L.J.; Beraldo, H.; Valdés-Martínez, J.; Hernández-Ortega, S.; West, D.X. J. Mol. Struct. 1999, 510, 157-163; [ Links ] West, D. X.; Castiñeiras, A.; Bermejo, E. J. Mol. Struct. 2000, 520, 103-106; [ Links ]Swearingen, J.K.; West, D. X. Transition Metal Chemistry, 2001, 26, 252-260. [ Links ]
10. Valdés-Martínez, J.; Hernández-Ortega, S.; West, D.X.; Ackerman, L.J.; Swearingen, J.K.; Hermetet, A.K. J. Mol. Struct. 1999, 478, 219-226; [ Links ] West, D. X.; Hermetet, A. K.; Ackerman, L. J.; Valdés-Martínez J.; Hernández-Ortega, S.; Acta Cryst. 1999, C55, 811-813; [ Links ] West, D. X.; Swearingen, J.K.; Hermetet, A. K.; Ackerman L.J.; Presto, C. J. Mol. Struct. 2000, 522, 27-36; [ Links ] Valdés-Martínez, J.; Hernández-Ortega, S.; Ackerman, L.J.; Le, D. T.; Swearingen J.K.; and West, D. X. J. Mol. Struct. 2000, 524, 51-59; [ Links ] West, D.X.; Swearingen, J. K.; Hermetet, A. K.; Ackerman. L.J.; J. Mol. Struct. 2001, 95-105. [ Links ]
11. West, D.X.; Nassar, A.A.; El-Saied, F.A.; Ayad, M.I. Transition Met. Chem. 1998, 23, 321-325. [ Links ]
12. West, D.X.; Nassar, A.A.; El-Saied, F.A.; Ayad, M.I.; Transition Met. Chem. 1998, 23, 423-427. [ Links ]
13. West, D.X.; Nassar, A.A.; El-Saied, F.A.; Ayad, M.I.; Transition Met. Chem. 1999, 24, 617-621. [ Links ]
14. Allen, F.H., Kennard, O., Watson, D.G., Brammer, L. and Orpen, A.G., J. Chem. Soc. Perkin Trans. II 1987, S1-S19. [ Links ]
15. Scovill, J. P. Phosphorus, Sulfur, and Silicon 1991, 60, 15-19. [ Links ]
16. Sheldrick, G.M. SHELX-97. Program for the Refinement of Crystal Structures, 1997, University of Göttingen, Germany. [ Links ]
17. International Tables for X-ray Crystallography, 1995, Vol. C, Kluwer Academic Publishers, Dordrecht, The Netherlands. [ Links ]
18. Spek, A.L. PLATON. A Multipurpose Crystallographic Tool, 1999, Utrecht University, Utrecht, The Netherlands. [ Links ]














