Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Sociedad Química de México
versión impresa ISSN 0583-7693
Rev. Soc. Quím. Méx vol.46 no.3 Ciudad de México jul./sep. 2002
Investigación
Purification and Properties of an Extracellular Halophilic Serine-Protease from Haloferax mediterranei
Héctor Nolasco1,2*, Donald J. Kushner1,3, and José-Luis Ochoa2
1 Department of Biology, University of Ottawa, Ottawa, Ontario K1N 6N5, Canada.
2 Lab. Comparative Biochemistry and Physiology. Centro de Investigaciones Biológicas del Noroeste, Apdo. Postal 128, La Paz, Baja California Sur, 23000, México. Tel: (612) 125-36-33; Fax: (612) 125-36-25. E-mail: hnolasco@cibnor.mx
3 Present address: Department of Microbiology, University of Toronto, Toronto, Ontario M5S 1A8, Canada.
Recibido el 16 de enero del 2002.
Aceptado el 27 de mayo del 2002.
Abstract
Na+, K+, and Mg+2 ions are essential for Haloferax mediterranei growth and extra-cellular protease production. Ammonium ions (> 2 % w/v) reduce enzyme production, whereas Ca+2 ions (~10 mM) enhance it and, in addition, activate the enzyme. The extra-cellular enzyme purified by ultra-filtration and Hydrophobic Interaction Chromatography, consists of a single polypeptide chain of MW 26 500 Da, with optimum activity at 3 M NaCl and pH 8 within 45 to 50 °C. This enzyme belongs to the serine-protease class and could be useful in a variety of industrial applications.
Keywords: Haloferax mediterranei, extracellular, protease, serine-proteases.
Resumen
Na+, K+, y Mg+2 son iones esenciales para el cultivo y la producción de la proteasa extracelular de Haloferax mediterranei. Los iones amonio (> 2 % m/v) reducen la producción de enzima, en cambio los de Ca+2 (~10 mM) la favorecen y, además, activan la enzima. La enzima extracelular, purificada mediante ultra-filtración y Cromatografía de Interacción Hidrofóbica, consiste en una cadena de polipéptido de PM 25,500 Da, con una actividad óptima a NaCl 3 M, pH 8, dentro del intervalo de 45-50 °C. Esta enzima pertenece a la clase de serina-proteasas que pueden encontrar atractivas aplicaciones industriales.
Palabras clave: Haloferax mediterranei, proteasa extracelular, serin-proteasa.
Dedicated to Dr. Barbarín Arreguín Lozano¶
Introduction
The halophilic microorganisms, represented by the halobacteria (extremely halophilic aerobic Archaeabacteria), the moderate halophiles (bacteria and some methanogens), and several eukaryotic algae, and their products, have been a research subject in modern biotechnology [1]. The halobacteria in particular, which are organisms usually found in habitats where the salt concentration is higher than sea water [2-4], seem to be able to produce a number of metabolites and polymers such as polyhydroxybutirate (PHB) [5], sulfated polysaccharides [6], and cell wall components [7], of industrial and biomedical interest. Haloferax mediterranei, first isolated by Rodríguez-Valera et al. [3], and later reassigned by Torreblanca et al. [8], has been found to be an excellent producer of PHB [9], and extracellular sulfated polysaccharides [6] that may have attractive applications as biodegradable polymers and anti-adhesives. Hf. mediterranei, considered a fast-growing species, has been used as a strain model in many studies and biotechnological applications [10, 11]. To date, several enzymes from Hf. mediterranei have been isolated, purified, and characterized [12-19], yet, the one responsible for its ability to hydrolyze casein and gelatin in solid media [3, 4] has not been described due to technical difficulties for isolating an active form of the enzyme in a pure state [2, 20-25].
As with most halophilic enzymes, the extracellular proteolytic activity of Hf. mediterranei looses activity when exposed to low salt concentrations [21, 26-32], and not even the use of enzyme substrates and cofactors to prevent inactivation [27-29, 33, 34] has been successful [20, 21]. Being aware of the advantages offered by Hydrophobic Interaction Chromatography, a technique that allows the use of a high salt concentration to favor a selective adsorption of the protein on the basis of its hydrophobicity [35], we decided to explore its application in the isolation of the extracellular enzyme responsible of the proteolytic activity shown by Hf. mediterranei in solid culture media containing casein or gelatin. Accordingly, and based on previous reports [36-40], we studied first the hydrolytic enzyme properties of the crude culture supernatant under different conditions to identify an optimum procedure for its purification and, afterwards, the properties of the active purified enzyme.
Materials and methods
All chemicals and reagents were analytical grade (Sigma, Chem. Co., St. Louis, MO, USA). Unless indicated otherwise, all solutions and culture media were prepared in 0.1 M Tris-HCl, pH 8.0.
Organism and culture conditions. Haloferax mediterranei (ATCC 33500) was kindly donated by Dr. Rodríguez-Valera of the University of Alicante in Spain. Five mL of a thick cell suspension (A580 nm > 2) were washed by repeated centrifugation and resuspension in 5 mL of 20 % seawater-salts solution (SWS) with the following composition (%, w/v): 15.6 % NaCl, 2.0 % MgSO4.7H2O, 1.3 % MgCl2. 6H20, 0.4 % KCl, 0.1% CaCl2. 6H20, 0.02 % NaHCO3, 0.08 % NaBr, and 0.05 % KH2PO4. The washed cells in 5 mL of 20 % SWS were used to inoculate 1000 mL of 20 % SWS supplemented with 1 % glucose and 0.2 % NH4Cl (This medium will be referred to as 20 % SWGA) in 2.8-L Fernbach flasks. Incubation was carried out at 37 °C, 7 days, under constant stirring (120 rpm). The corresponding culture supernatant (CS) was collected by centrifugation at 10 °C, 13,000 × g, for 30 min (Beckman ultracentrifuge L7-55).
Enzyme assay. Casein digestion was carried out following the method described by Norberg and Hofsten [36], using 1 mL of 1 % (w/v) casein (Hammarsten, BDH Chemicals, Poole, England) in 20 % SWS-Tris buffer (0.1 mM, pH 8.0) mixed with 0.5 mL of the enzyme preparation. After incubation for 30 min at 38 °C, the reaction was stopped by addition of 3 mL of 5 % (w/v) trichloroacetic acid (TCA). The precipitated proteins were removed by centrifugation (15,000 × g, 5 min, room temperature) and the supernatant absorbance at 280 nm determined. The reference cell contained the supernatant of a mixture of 1 mL of casein substrate, 3 ml of 5 % TCA, and 0.5 mL of CS, added in that order. Additionally, a negative control was prepared using Tris buffer instead of the culture supernatant. Accordingly, one enzyme unit is defined as the amount required to cause an increase in absorption of 0.01 OD units per minute at 280 nm.
Effect of salt concentration on CS proteolytic activity. The remaining CS was concentrated about 20 times in an Amicon apparatus (Mod. 202) using a diaflomembrane YM10. The new concentrated material was designated as CS10. One mL of CS10 was diluted 1:20 with Tris buffer (0.1 M, pH 8.0) and the proteolytic activity measured as above.
Cation effect on CS proteolytic activity. To determine the specific cation requirements for protease activity, 5 mL of CS10 were dialyzed against 2000 mL of different 20 % SWS solutions lacking one of the following cations Na+, K+, Mg2+, or Ca2+, for 48 h at 4 °C. After dialysis, the remaining protease activity was measured as above using a 1:10 dilution of the dialyzed sample with the corresponding dialysis solution. The control consisted of a CS10 sample treated in the same way but dialyzed and diluted with 20% SWS.
Optimum sodium chloride concentration for stability of CS proteolytic activity. To determine the optimum NaCl concentration required for CS protease stability, two different experiments were carried out:
1. One mL of CS10 was dialyzed against 1000 mL of different NaCl solutions (2.5, 5, 7.5, 10, and 15.6%) containing 6.8 mM CaCl2, at 4 °C for 24 h. The dialyzed samples were diluted 1:10 with 2.67 M NaCl containing 6.8 mM CaCl2, and the protease activity measured using 0.5 mL of enzyme solution adjusted to 0.89 M NaCl.
2. One mL of CS10 was diluted 1:30 with different NaCl concentrations (2.5, 5, 7.5, 10, 15, 20, 25, and 30 % [w/v]) containing 6.8 mM CaCl2 and incubated at 4 °C for 72 h. After this period, the proteolytic activity was found using 0.5 mL of the treated enzyme solution adjusted to a final NaCl and CaCl2 concentrations of 2.56 M and 2.2 mM, respectively.
Specific ion requirement for CS proteolytic activity. To determine the type of salt required for protease stability, samples of 1 mL of CS10, previously diluted 1: 10 in 20 % SWS, were dialyzed against 100 mL of different 2.67 M, or saturated, solutions of CuCl2, LiCl, CsCl, KCl, NH4Cl, NaF, NaHCO3, NaH2PO4, Na2SO4, NaBr, NaNO3, (NH4)2SO4, sodium citrate, sodium lactate, sodium acetate, sodium formate, sodium succinate, sucrose, betaine, glycerol, and Tris buffer, for 6 h at room temperature, and then 36 h at 4 °C. The samples were finally dialyzed against 15.6 % (equivalent to 2.67 M) NaCl solution containing 6.8 mM CaCl2 solution for 36 h at 4 °C and the proteolytic activity determined.
Chromatographic assays. All experiments were made at room temperature on Phenyl Sepharose Cl-4B (Amersham-Pharmacia Biotech, Uppsala, Swden) and all solutions prepared in Tris-HCl buffer (0.1 M, pH 8.0):
1. A sample of 0.6 mL of CS10 was applied to a 1.8 mL of gel bed column previously equilibrated with the starting solution consisting of 2.67 M NaCl and 10 mM CaCl2. After washing the column with 2 volumes of starting buffer, a decreasing linear gradient of NaCl (from 2.67 to 0 M) and an increasing gradient of ethylene glycol (from 0 to 50 %) were used for elution. The flow rate was approximately 15 mL / h and fractions of 0.5 mL were collected in 1.5 mL of 20 % SWS.
2. One mL of CS10 diluted 1:1 in 5 M NaCl, was applied to a 5 mL gel bed column equilibrated as above. A decreasing linear gradient of NaCl (from 5.0 to 0 M) and an increasing gradient of ethylene glycol (from 0 to 50 %) were used for elution. The flow rate in this case was 26 mL / h and 2 mL fractions were collected in 2 mL of 5 M NaCl.
3. A sample of 0.5 mL of CS10 diluted 1:1 in 5 M NaCl, was applied to a column of 1.8 mL of gel bed equilibrated as above and the elution carried out with a NaCl decreasing gradient. The flow rate was 13 mL / h and fractions of 1 mL collected in 1 mL of 5 M NaCl.
CS Protease purification. All steps were done at room temperature as follows:
1. Ultrafiltration. A fresh culture supernatant was subjected to ultrafiltration in an Amicon system using a YM100 diaflomembrane to separate the high molecular weight exopolysaccharide [41, 42]. The filtrate was then concentrated (1/20 of the original volume) using a YM10 membrane. This concentrated protease material free of exopolysaccharides was designated as CS-EP.
2. Hydrophobic interaction chromatography. A 4 mL sample of CS-EP, dialyzed 48 h at 4 °C against 5 M NaCl-10 mM CaCl2 in Tris-HCl 0.1 M, pH 8.0 buffer, was applied to a column (2.6 × 40 cm) packed with 100 mL of Phenyl Sepharose Cl-4B, equilibrated with the dialysis buffer. The column was washed with 150 mL of the starting buffer and eluted with a decreasing sodium chloride linear gradient at a flow rate of 28 mL / h. Fractions of 6 mL were collected in tubes containing 5 mL of 5 M NaCl and assayed for protein [43] and protease activity.
3. Electrophoresis. SDS-polyacrylamide gel electrophoresis (SDS-PAGE) was done as described by Laemmli [44]. The gels (10 % Polyacrylamide, PAA), were cast in 3-mm slabs in a 12-cm vertical electrophoresis apparatus (Bio-Rad laboratories, Richmond, Ca, USA). Samples and molecular weight markers (Dalton Mark VI, Sigma Chemicals, St. Louis Mo. USA) were prepared in SDS-reducing buffer containing 0.125M Tris-HCl, pH 8.0), 1 % (w/v) SDS, 5 % (v/v) 2-mercaptoethanol, 0.29 % (w/v) EDTA, and 25 % (w/v) sucrose, and immediately heated at 100 °C for 5 min. Electrophoresis was done at a constant voltage (120 V) until the tracking dye (bromophenol blue) reached 1 cm from the bottom of the gel.
Optimum salt concentration, pH, and temperature studies on enzyme activity of the purified protease
1. Using Black Gelatin (ENZYMMIS, Czechoslovakia, kindly donated by Dr. Ivo Safarik) as substrate, the optimum salt (NaCl) concentration for enzyme activity was determined varying the amount of NaCl in the assay mixture (0 to 4.0 M). The procedure consisted in homogenizing 20 mg of the insoluble substrate in 2 mL of Tris-HCl (0.1 M, pH 8.0) containing different concentrations of NaCl and after 10 min of preincubation at 38 °C, 10 µL of a purified enzyme stock solution added and the incubation prolonged for 15 min with occasional stirring. The reaction was ended removing the insoluble material by filtration through a Whatman filter paper and the absorbance at 850 nm measured against the corresponding blank.
2. The enzyme stability at different NaCl concentrations was determined as follows: Ten µL of purified protease stock solution was mixed with 640 µL of a NaCl solution of different concentration (0, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, and 5.0 M) and incubated at 38oC for 60 min. After incubation, the NaCl concentration was adjusted to 2.0 M using different proportions of Tris and / or 5M NaCl to a final volume of 1300 µL. Two hundred µL of a 5 % (w/v) casein substrate was added to determine enzyme activity after 30 min of incubation at 38 °C. The optical density of the relative enzyme activity was found as above.
3. The effect of pH on enzyme activity was determined adding 10 µL of the purified enzyme stock solution to a mixture containing 200 µL of 5 % (w/v) casein in distilled water, 200 µL of 5 M NaCl-10 mM CaCl2, and 1090 µL of 0.1 M glycine buffer with the pH adjusted between 3 and 12 with 1.0 M HCl or 1.0 M NaOH, as necessary (except for pH 6.5, 7, 7.5 and 8, were 0.1 M Tris-HCl buffer was used). The enzyme activity was then determined as above.
4. The stability of the enzyme towards the pH of the medium was found by exposing 10 µL of the enzyme stock solution to 490 µL of the corresponding pH solution at a final concentration of 2.5 M NaCl and 5 mM CaCl2. After incubating for 18 h, at 4 oC, activity was determined using 1 mL of 1 % (w/v) casein prepared in 0.3 M Tris buffer (pH 8.0).
5. The effect of temperature on enzyme activity was determined as follows: One mL of 1 % (w/v) casein substrate warmed at different temperatures (25 to 100 °C) was mixed with 490 µL of 20 % SWS at the corresponding temperature. Then 10 µL of enzyme stock solution were added and the activity measured after 15 min incubation.
6. To determine the effect of temperature on enzyme stability, 10 µL of purified enzyme stock solution were mixed with 490 µL of 20 % SWS at different temperatures (35 to 100 oC). After 10 min, the solutions were quickly chilled in an ice bath. The enzyme activity was measured for 15 min near the optimum temperature (45 °C) using 1 mL of 1 % (w/v) casein substrate.
7. The effect of NaCl concentration on enzyme stability at high temperature was measured as follows: 10 µL of purified enzyme stock solution was mixed with 640 µL of ice cold NaCl solution (1.5 to 5.0 M), then immediately exposed to 68 °C for 10 min and quickly cooled in an ice bath. The NaCl concentration was adjusted to 2.0 M in a final volume of 1,300 µL. The activity was then measured for 30 min near the optimum temperature (45 °C), using 200 µL of 5 % (w/v) casein substrate.
8. The temperature and salt concentration effect on enzyme activity was determined as follows: 200 µL of 5 % (w/v) casein substrate were mixed with 1,290 µL of one of the following NaCl solutions: 1, 2, 3, and 4 M (final concentration in the assay mixture was 0.86, 1.72, 2.58, and 3.44 M NaCl) at temperatures of 45 to 85 °C. Then, 10 µL of the purified enzyme stock solution was added and the activity measured at the corresponding temperature after 15 min incubation.
9. The effect of the different salts (listed in Table 2) on the activity of the purified enzyme fraction was found using 1290 µL of salt solution and 200 µL of 5% (w/v) casein with 10 µL of the purified enzyme stock solution. The activity was determined as above.
10. The effect of exposure of the protease to different chemicals (listed in Table 3) was studied by first incubating 10 µL of the purified enzyme stock solution with 490 µL of the chemical solution for 60 min at 38 °C. After incubation, 800 µL of the buffer solution containing 2.66 M NaCl and 5.3 mM of CaCl2 (in Tris-HCl, 0.1 M, pH 8.0) were added, and the remaining activity was measured in the presence of 200 µL of 5 % (w/v) casein substrate.
11. To determine the effect of chelating agents, such as EDTA, and of various protease inhibitors, like Trypsin, serine-protease PMSFM, and Sulfhydryl protease inhibitors, 10 µL of the purified enzyme stock solution were mixed with 5 to 25 µL of the various agents at the concentration listed in Table 5. The mixture volume was adjusted to 500 µL with 5.0 M NaCl and incubated for 30 min to estimate the enzyme activity as above.
12. The Ca+2 concentration required to activate the enzyme was determined by mixing 10 µL of the purified enzyme stock solution with 9.9 mM EDTA in 400 µL of Tris. Immediately, different amounts of CaCl2 were added and the volume adjusted to 500 µL to a final concentration of 0 -24.9 mM CaCl2. The incubation was continued for 30 min at 37 °C and the enzyme activity determined with 1 mL of 1 % (w/v) casein solution as above.
Results
Hf. mediterranei growth and extracellular proteolytic activity at various salt concentrations
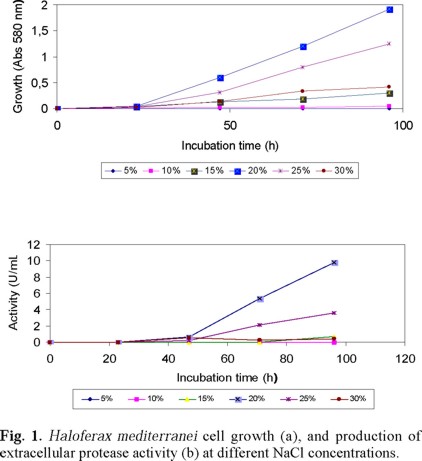
As expected, Hf. mediterranei was able to grow and secrete a substance with proteolytic activity when cultured in glucose: NH4Cl (1 %: 0.2 % [w/v]) in 20 % SWGA medium, as a function of salt concentration (Figs. 1a and 1b). The maximum optical density after 4 days of incubation was 1.9 and the proteolytic activity of the supernatant 9 U / mL at 3.4 M (20 %) NaCl. Also the concentration of ammonium ions affected cell growth and the extracellular enzyme activity of Hf. mediterranei, being 1.5 % optimal for cell growth (Fig. 2a), and 0.2 % for enzyme activity (Fig. 2b) in culture media prepared in 20 % SWS. Hence, the following experiments were run with 0.2 % NH4Cl, 20 % SWS, in order to obtain maximum enzyme activity and growth.

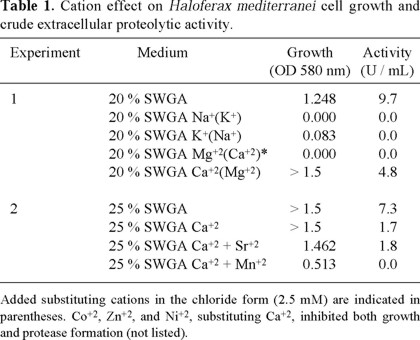
Ion-specificity requirements for Hf. mediterranei growth and protease production
Table 1 shows the effect of different cations on Hf. mediterranei cell growth and on its extra-cellular protease production. When Na+ was replaced by K+, no cell growth, and consequently no proteolytic activity in the culture medium was detected. When K+ was replaced with Na+, a slight cell biomass increase without extracellular proteolytic activity was observed. When Ca+2 was replaced by Mg+2, a considerable increase in cell biomass was observed, but the number of protease units / mL was half of that with 20% SWGA medium. On the other hand, when Mg+2 was substituted by Ca+2, neither cell growth nor enzyme activity in the medium could be observed. In conclusion, according to these results Na+, K+, and Mg+2 present in 20 % SWGA medium appear to be essential for Hf. mediterranei cell growth and extracellular proteolytic enzyme production. A higher salt concentration (25 % SWGA) with concomitant increase in concentration of the corresponding ions, did improve the cell biomass yield but not the enzyme activity. Therefore, the 20 % SWGA medium was considered suitable for the production of biomass and extracellular enzyme activity in the following experiments.
Stability of extracellular protease activity in different chemical conditions
In order to determine the appropriate experimental conditions for purification of Hf. mediterranei extracellular hydrolytic enzyme, the culture supernatant obtained in 20 % SWGA medium was concentrated 20 times by ultrafiltration and the enzyme activity assayed after dilution (1:20) with Tris buffer (0.1 M, pH 8.0). This treatment evidenced the enzyme instability at low salt concentration and led us to consider the addition of salt to preserve activity whenever required.
Dialysis of CS10 against different 20 % SWS in which the cations were removed or exchanged (i.e. Na+ vs. K+, and Ca2+ vs. Mg2+), revealed the enzyme susceptibility towards the different cation species. For example, without sodium ions, a complete loss of enzyme activity was noticed (Table 2). Hence, considering the results of Table 1 discussed above, Na+ was not only important for extracellular enzyme production but also for its activity. In contrast, despite the previous observation suggesting that K+ was essential for growth and extracellular enzyme production, the enzyme was fully active in the absence of potassium ions, indicating that K+ was not essential for enzyme activity. Dialysis of CS10 against 20 % SWS without specific divalent cations on the other hand, revealed that the extracellular enzyme activity of Hf. mediterranei was still high in the absence of Mg2+ (95 % of the original activity in 20 % SWS) and significantly reduced in the absence of Ca2+ (67 % of the original activity in 20 % SWS). Thus, it may be concluded that the most important cations for Hf. mediterranei extracellular proteolytic activity are Na+ and Ca2+ ions.
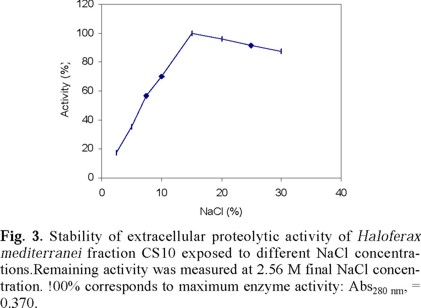
The effect of NaCl concentration on Hf. mediterranei extracellular enzyme activity is shown in Fig. 3. The maximum recovered activity assayed at 2.67 M NaCl after exposure to different salt concentrations was observed at 17 % w/v NaCl. Exposure to lower or higher salt concentration reduced enzyme activity.
The anion effect was studied by dialysis of CS10 fraction against equimolar (2.67 M), or saturated solutions, of different compounds (Table 3). Modification of protease activity in this case, evidenced the influence of the anion species in enzyme activity. For example, NaF, NaHCO3, NaH2PO4, and NaNO3, provoked a complete enzyme inhibition, while sodium citrate > sodium acetate > sodium formate > sodium succinate > sodium lactate, derivatives, showed a decreasing inhibition power (in that order). Saturated solutions of sucrose, and betaine, and 5.20 M glycerol, on the other hand, provided an excellent enzyme protection allowing a 97-99 % activity recovery.
It should be borne on mind that Hf. mediterranei is also capable of excreting polysaccharides and other substances that may participate in the overall enzyme stabilization process. The exopolysaccharide for example, may play an important role in controlling water activity, which can be also modified by the presence of salts according to the nature of the ions involved.
The above experiments provided important information about the conditions required for the proper handling of an active extracellular enzyme produced by Hf. meditarranei.
Once established, we were able to introduce the following method for its isolation and purification: First, for the removal of the exopolysaccharide component a selective ultrafiltration procedure was carried out. Then, taking into account the nature (extracellular), and composition (high salt concentration culture medium) of the CS10-EP preparation, Hydrophobic Interaction Chromatography, HIC, was considered the method of choice for the enzyme purification as shown in the following section.
Chromatographic assays
Fig. 4 shows the separation profile of the different protein fractions contained in a CS10-EP sample applied to a Phenyl Sepharose column and eluted with a decreasing NaCl concentration gradient. The major peaks, containing also most of the proteolytic activity, were pooled after the corresponding electophoretic analysis revealed no differences in protein pattern between the fractions. Yet, under such chromatographic conditions, pooling yielded only 25 % of the original activity. A large improvement in enzyme recovery was achieved when the chromatography was carried out in a column equilibrated with 2.6 M NaCl-10 mM CaCl2, and a decreasing salt gradient for elution. In this case the yield of proteolytic activity was nearly 100 %. The active fractions that separately emerged from the column (Fig. 4) correspond to the same protein, as evidenced by SDS-PAGE electrophoresis analysis, and this separation can be accounted on the basis of the column heterogeneity, which may adsorb a given protein with different strengths. The increased recovery obtained by adding Ca2+ simply reflects the importance of this cation on enzyme activity and stability (discussed previously).

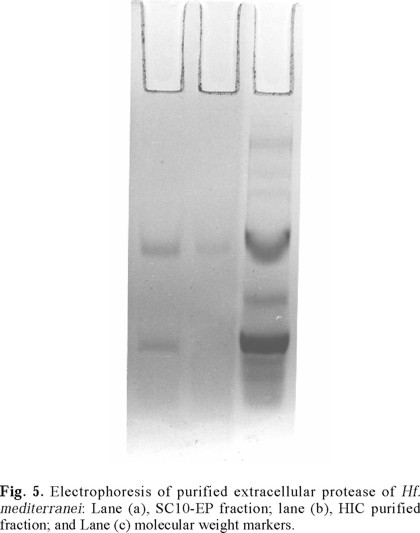
The SDS-PAGE electrophoretic analysis of the active purified fraction obtained by HIC (Fig. 5), reinforces the above assumption of the presence of a single protein band fraction of about 26 500 MW responsible of the enzyme activity. Because the employed electrophoresis conditions are known to promote protein disgregation, and because only one protein band in the gel was observed, we have concluded that the extracellular protease of Hf. mediterranei consists of a single polypeptide chain which purification summary is shown in Table 4.
Properties of the purified protease
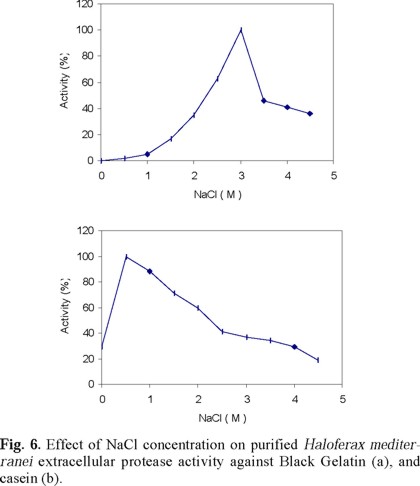
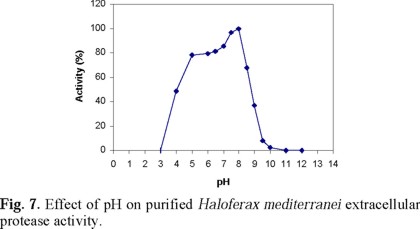
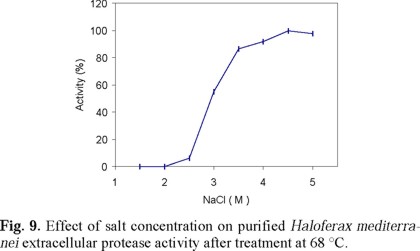
Table 3 describes the effect of different ions on the activity of the purified extracellular protease of Hf. mediterranei, Figures 6, 7 and 8 show the effect of salt concentration, pH, and temperature, on enzyme activity. The combined effect of such parameters is presented in Figs. 9 and 10 and, finally, the effect of various protease inhibitors in Table 5. All these tests provided some useful information about the properties of the enzyme that led us to conclude that the extracellular enzyme activity of Hf. mediterranei corresponds to a halophilic serine-protease.


Discussion
Enzymes from halophilic archaebacteria are highly unstable at low salt concentrations of neutral salts [45]. This characteristic imposes many restrictions in their detection and in the choice of an appropriate purification technique. Although some halophilic enzymes may be reactivated from the salt-free solutions [21, 26-32], and this facilitates their purification using standard methods, i.e. ion-exchange chromatography, many others cannot [26, 27].
Previous reports on the purification of halophilic enzymes from Hf. meditarranei refer mostly to cytoplasmic or membrane-bound proteins [12, 19], in which cases the enzyme activity decreases with increasing salt concentration in the molar range. In our case, the extra-cellular protease stability and activity of Hf. mediterranei are dependent of the salt concentration in the medium and appear favored at concentrations within 2.56 and 4 M NaCl range.
Most reports of halophilic enzyme isolation and purification dealt well with enzyme stability in different salts like sodium chloride, potassium chloride, and ammonium sulfate. Hence, reported purification methods in those cases have included: ammonium sulfate fractionation, salting out chromatography, ion exchange chromatography, gel filtration, affinity chromatography, and chromatography on hydroxylapatite [20, 21, 24, 25, 28-30, 32, 33, 37, 38, 40, 46-58]. Our study, however, has shown that the extracellular protease activity of Hf. mediterranei is highly unstable and irreversibly lost at low salt concentration. Moreover, Hf. mediterranei extracellular proteolytic enzyme requires Na+ and Ca2+ to express activity. These features made the application of the above purification techniques inadequate and, therefore, an alternative procedure had to be explored. Hydrophobic Interaction Chromatography, HIC, has been seldom applied in the purification of halophilic enzymes [21, 39, 40, 49], in spite of its inherent advantage of combining gel hydrophobicity and salt concentration for the adsorption-elution phenomenon, that helps to resolve the separation of biomolecules on the basis of their hydrophobicity [35]. The technique has been successfully applied, for example, by Kamekura and Seno [39] who were able to purify an extracellular protease from an unidentified strain of a halophilic archaebacterium on Butyl Toyopearl and Phenyl Sepharose. Unfortunately, they were not successful in the isolation and purification of a similar enzyme from Hf. mediterranei. We may ascribe that failure to inappropriate experimental conditions to preserve enzyme activity.
In our study we found that exposure of the culture supernatant of Hf. Mediterranei to different kinds of salts, including ammonium sulfate, resulted in a considerable loss of activity. Hence, the traditional salting-out protein purification and concentration procedure cannot be applied in this case. We also found a great loss of activity when the culture supernatant CS10-EP was kept at room temperature, and that the protease activity yield could be enhanced if the purification process was carried at low temperature; presumably, due to a reduced autohydrolysis. Finally, we found that CS storing below 0 °C helps to preserve its activity for at least 6 months.
Because Hf. mediterranei strain 33500 can grow and produce extracellular-proteolytic activity when cultured aerobically in different media, such as glucose-ammonium chloride-based medium with 20 % SWS, we have concluded that this is a constitutive property of Hf. mediterranei. We have also confirmed that Na+, K+, and Mg+2 ions are essential for Hf. mediterranei growth and protease production, as occurs with other Archaebacteria [59], and that Ca+2 ions are apparently important for enzyme activity but not for cell growth or enzyme production, as in the case of Hf. volcanii and Halobacterium (now Haloarcula) morismortui [60], and the strain 172 PI [39], but in contrast to Hb. cutirubrum [61], Hb. halobium [38], and to some other marine bacteria [62], where Ca+2 ions are essential for both enzyme production and activity.
As with most halophilic enzymes [63], the purified protease of Hf. mediterranei is completely inactivated at low salt concentration. This denaturation is irreversible, as observed also with other halophilic archaebacteria [36, 38, 39]. The purified extracellular protease activity of Hf. mediterranei shows a marked specificity for NaCl, rather than for KCl for activity, as does the protease from Hb. salinarum [36], which is compatible with the notion that KCl is more important internally than externally for cell function [64]. Nevertheless, there are some halophilic proteases, like the one from Hb. halobium, which have been shown to be stable and active in the presence of either NaCl or KCl [38].
Extracellular Hf. mediterranei protease shows an optimum activity at about 3 M NaCl against Black Gelatin, thus showing a lower NaCl requirement for maximum activity than Hb. halobium [38], and 172 P1 proteases [39], with this substrate. However, with casein Hf. mediterranei protease retained activity even at low salt concentrations. Such behavior was similar to that of another Hb. mediterranei strain [40] and also of 172 P1 protease [39], which have been shown to be more salt dependent when acting on short, synthetic succinyl peptides. The effect of salt concentration on both substrate and enzyme may favor their association and therefore help the enzyme function better. For casein, Hf. mediterranei extracellular enzyme showed an optimum activity at 0.5 M NaCl, suggesting that this substrate may protect the enzyme because, as we have pointed out, the purified protease without casein is quickly inactivated in NaCl concentrations lower than 1 M.
The maximum activity for purified Hf. mediterranei protease was found at 6.0 to 8.0 pH range similarly to Hb. salinarum [36] and Hb. halobium [38], and lower than Hb. mediterranei strain 1538 [40], and of strain 172 P1 protease [39]. On the other hand, Hf. mediterranei protease was found to be stable over a broad pH range (Fig. 7).
Like the 172 P1 protease [39], Hf. mediterranei protease showed maximum activity around 50 °C (using casein as a substrate at low salt concentration), increasing with salt concentration up to 55 °C (Fig. 8). A similar optimum temperature was reported for the protease activity of Hb. mediterranei strain 1538 on a small synthetic polypeptide [40]. In general, Hf. mediterranei protease follows the same pattern as other halophilic enzymes showing a close relationship between temperature stability and salt concentration [(Fig. 9); Refs. 25, 65].
Hf. mediterranei protease strain 33500 is totally inhibited by PMSF (Table 5) suggesting that it is a serine-protease, like the protease of Hb. halobium [38], of 72 P1 [39], and Hb. mediterranei strain 1538 [40]. It is also inhibited by EDTA and reactivated by Ca+2 ions, which indicates its metallo-protein nature, in contrast to the serine protease of Hb. mediterranei strain 1538, which is not inhibited by EDTA. In addition, the Hf. mediterranei strain 33500 protease shows a different molecular weight (26,500) than that of Hb. mediterranei strain 1538 (41, 000) [40].
The extracellular Hf. mediterranei protease has a specific NaCl requirement for activity and stability (Tables 1, 2). KCl and other salts, including ammonium salts, were much less efficient The specific requirement of NaCl for enzyme activity and stability (Fig. 9), as with other halophilic archaebacteria [59], is not surprising if we consider the ionic composition of Hf. mediterranei habitat, in which the main salt is NaCl [59]. The capability of Hf. mediterranei protease to be active in high solute concentrations may be a useful property for protein hydrolysis in processes with high solute concentrations and low water activity.
Acknowledgements
This work was partly supported by a grant to DJK from the NSERC of Canada, and from the Government of Canada Awards Program to HN. We thank Dr. Alicia Espinosa-Lara, M.Sc. Elena Irma Villarreal Moguel, and Dr. Ramón Cruz-Camarillo, for fruitful discussions with HN during his PhD thesis work.
References
1. Margesin, R.; Schinner, F. Extremophiles, 2001, 5,73-83. [ Links ]
2. Kushner, D.J. The halobacteriaceae. In: Woese, C.R.; Wolfe, R.S. (eds.). The Bacteria. Academic Press, Orlando, 1985, 8, 171-214. [ Links ]
3. Rodríguez-Valera, F.; Ruíz-Barranquero, F.; Ramos-Comerzana, A. J. Gen. Microbiol. 1980, 119, 535-538. [ Links ]
4. Rodríguez-Valera, F.; Juez, G.; Kushner, D. J. Syst. Appl. Microbiol. 1983, 4, 369-381. [ Links ]
5. Fernández-Castillo, R.; Rodríguez-Valera, F.; González-Ramos, J.; Ruiz-Barranquero, F. Appl. Environm. Microbiol. 1986, 51, 214-216. [ Links ]
6. Parolis, H.; Parolis, L.A.; Boan, I.F.; Rodríguez-Valera, F.; Widmalm, G.; Manca, M.C.; Jansson, P.E.; Sutherland, I.W. J. Carbohydr. Res. 1996, 295, 147-156. [ Links ]
7. Baumgarten, J.; Brunner, H.; Hildebrand, H.; Piel, N.; Sperzel, M. Pat. US5091364. 1992. [ Links ]
8. Torreblanca, M.; Rodríguez-Valera, F.; Juez, G.; Ventosa, A.; Kamekura, M.; Kushner, D.J. Syst. Appl. Microbiol. 1986, 8, 89-99. [ Links ]
9. Rodríguez-Valera, F.; García-Lillo, J.A. Halobacteria as producers of polyhydroxyalkanoates. In: Bacterial-Polyhydroxyalkanoates-Poly-beta-Hydroxyalkanoic acid (Schlegel, H.G.; Steinbuchel, A. eds.). 1992, 181-186. Germany. [ Links ]
10. Nieto, J.J.; Fernández-Castillo R.; Nagias, M.; Ruiz-Barranquero, F. Curr. Microbiol. 1992, 24, 41-47. [ Links ]
11. D'Souza, S.E.; Altekar, W.; D'Souza, S.F. J. Biochem. Biophys. Meth. 1992, 24, 239-247. [ Links ]
12. Bonete, M.J.; Pire, C.; Llorca, F.L.; Camacho, M.L. FEBS Lett. 1996, 383, 227-229. [ Links ]
13. Bonete, M.J.; Ferrer, J.; Pire, C.; Penades, M.; Ruiz, J.L. Biochemie, 2000, 82, 1143-1150. [ Links ]
14. Ferrer, J; Pérez-Pomares, F.; Bonete, M.J. FEMS Microbiol. Lett. 1996, 15, 59-63. [ Links ]
15. Ferrer, J.; Fisher, M.; Burke, J.; Sedelnikova, S.E.; Baker, P.J.; Gilmour, D.J.; Bonete, M.J.; Pire, C.; Esclapez, J.; Rice, D.W. Acta Crystallogr. D. Biol. Crystallogr. 2001, 57, 1998-1889. [ Links ]
16. Martínez-Espinoza, R.M.; Marhuenda-Egea, F.C.; Bonete, M.J. FEMS Microbiol. Lett. 2001, 204, 381-385. [ Links ]
17. Muriana, F.J.; Alvarez-Osorio, M.C.; Relimpio, A.M. Biochem. J. 1991, 15, 149-154. [ Links ]
18. Platas, G.; Meseguer, I.; Amils, R. Microbiologia, 1996, 12, 75-84. [ Links ]
19. Rajagopalan, R.; Altekar, W. Eur. J. Biochem. 1994, 221, 863-869. [ Links ]
20. Higa, A.I.; Cazzulo, J. J. Experientia, 1973, 29, 1081-1083. [ Links ]
21. Keradjopoulus, D.; Holldorf, A.W. Biochim. Biophys. Acta, 1979, 570, 1-10. [ Links ]
22. Baxter, R.M. Can. J. Microbiol. 1959, 5, 47-57. [ Links ]
23. Lanyi, J.K. Bacteriol. Rev. 1974, 38, 272-290. [ Links ]
24. Leicht, W. Eur. J. Biochem. 1978, 84, 33-139. [ Links ]
25. Leicht, W.; Werber, M.M.; Eisenberg, H. Biochemistry 1978, 17, 4004-4010. [ Links ]
26. Holmes, P.K.; Halvorson, H.O. J. Bacteriol. 1965a, 90, 312-315. [ Links ]
27. Holmes, P.K.; Halvorson, H.O. J. Bacteriol. 1965b, 90, 316-326. [ Links ]
28. Hubbard, J.S.; Miller, A.B. J. Bacteriol. 1969, 99, 161-168. [ Links ]
29. Hochstein, L.I.; Dalton, B.P. Biochim. Biophys. Acta 1973, 302, 16-228. [ Links ]
30. Kim, E.D.; Fitt, P.S. Biochem. J. 1977, 161, 313-320. [ Links ]
31. Keradjopoulos, D.; Holldorf, A.W. FEBS Lett. 1980, 112, 183-185. [ Links ]
32. Hallberg, C.; Hederstedt, L.; Baltscheffsky, H. Arch. Biochem. Biophys. 1985, 239, 200-205. [ Links ]
33. Mevarech, M.; Leicht, W.; Werber, M.M. Biochemistry 1976, 15, 2383-2386. [ Links ]
34. Pundak, S.; Aloni, H.; Eisenberg, H. Eur. J. Biochem. 1981, 118, 471-477. [ Links ]
35. Ochoa, J.L. Biochimie 1978, 60, 1-15. [ Links ]
36. Norberg, P.; Hofsten, B.V.; J. Gen. Microbiol. 1969, 55, 251-256. [ Links ]
37. Norberg, P.; Hofsten, B.V. J. Biochim. Biophys. Acta 1970, 220, 132-133. [ Links ]
38. Izotova, L.S.; Strongin, A.Y.; Chekulaeva, L.N.; Sterkin, V.E.; Ostoslavskaya, V.I.; Lyublinskaya, L.A.; Thimokhina, E.A.; Stepanov, V.M. J. Bacteriol. 1983, 155, 826-830. [ Links ]
39. Kamekura, M.; Seno, Y. Biochem Cell Biol. 1990, 68, 352-359. [ Links ]
40. Stepanov, V.M.; Rudenskaya, G.N.; Revina, L.P.; Gryaznova, Y.B.; Lysogarskaya E.N.; Filippova, I.Y.; Ivanova, I.I. Biochem. J. 1992, 285, 281-286. [ Links ]
41. Anton, J.; Mesenguer, I., Rodriguez-Valera, F. Appl. Environm. Microbiol. 1988, 54, 2381-2386. [ Links ]
42. Rodríguez-Valera, F.; García-Lillo, J. A.; Antón, J.; Meseguer, L.. Biopolymer production by Haloferax mediterranei. In: Rodríguez-Valera, F. (ed.) General and Applied aspects of Halophilic microorganisms. Plenum Press, New York. 1991. 402. [ Links ]
43. Bradford, M.M. Anal. Biochem. 1976, 72, 248-254. [ Links ]
44. Laemmli, U.K. Nature (London) 1970, 227, 680-685. [ Links ]
45. Madern, D.; Ebel, C.; Zaccai, G. Extremophiles 2000, 4, 91-98. [ Links ]
46. De Medicis, E.; Laliberte, J.F.; Vass-Marengo, J. Biochim. Biophys. Acta 1982, 708, 57-67. [ Links ]
47. Dundas, I.E. Eur. J. Biochem. 1970, 16, 393-398. [ Links ]
48. Fitt, P.S.; Badoo, P. Biochem. J. 1979, 181, 347-353. [ Links ]
49. Fukumori, Y.; Fujiwara, T.; Okada-Takahashi, T.; Mukohata, Y.; Yamanaka, T. J. Biochem. 1985, 98:1055-1061. [ Links ]
50. Greene, R.V.; MacDonald, R.E. Arch. Biochem. Biophys. 1984, 229, 576-584. [ Links ]
51. Kerscher, L.; Oesrterhelt, D. FEBS Lett. 1976, 67, 320-322. [ Links ]
52. Kerscher, L.; Oesterhelt, D. Eur. J. Biochem. 1981, 116, 587-594. [ Links ]
53. Koch-Schmidt, A.C.; Mosbach, K.; Werber, M.M. Eur. J. Biochem. 1979, 100, 213-218. [ Links ]
54. Hallberg, C.; Baltscheffky, H. FEBS Lett. 1981, 125, 201-204. [ Links ]
55. Leicht, W.; Pundak, S. Anal. Biochem. 1981, 114,186-192. [ Links ]
56. Mevarech, M.; Eisenberg, H.; Neumann, E. Biochemistry 1977, 16, 3781-3785. [ Links ]
57. Pundak, S.; Eisenberg, H. Eur. J. Biochem. 1981, 118, 463-470. [ Links ]
58. Werber, M.M.; Mevarech, M. Arch. Biochem. Biophys. 1978, 187, 447-456. [ Links ]
59. Kushner, D.J. Halophilic bacteria. In: Umbreit, W.W.; Pealman, D. (eds.). Advances in Applied Microbiology, Academic Press, London, 1985, 10, 73-99. [ Links ]
60. Cohen, S.; Oren, A.; Shilo, M. Arch. Microbiol. 1983, 136, 184-190. [ Links ]
61. Sehgal, S.N.; Gibbons, N.E. Can. J. Microbiol. 1960, 6, 165-169. [ Links ]
62. Sakata, T.; Kakimoto, D. Study of proteases of marine bacteria: Effect of cations on the enzyme production and activity. In: Saline Environment: Physiological and Biochemical Adaptation in Halophilic Microorganisms. (Morishita, H.; Masui, M. eds.). Business Centre for Academic Societies, Japan. 1980, 123-134. [ Links ]
63. Kushner, D.J. FEMS Microbiol. Rev. 1986, 39, 121-127. [ Links ]
64. Ginzburg, M.; Sachs, L.; Ginzburg, B.Z. J. Gen. Physiol. 1970, 55, 187-202. [ Links ]
65. Márquez, E.D.; Brodie, A.F. Biochim. Biophys. Acta. 1973, 321, 84-89. [ Links ]
Nota
¶This contribution is dedicated to honor the scientific career of doctor Barbarín Arreguín Lozano. Being a pioneer Plant Biochemist in Mexico, his scientific family tree now extends several generations (BA → JLO → HN → FV). We all try to be faithful following his example of devotion for science. We all enjoyed his teachings, friendship, and continuous support through all these years.