Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Sociedad Química de México
versión impresa ISSN 0583-7693
Rev. Soc. Quím. Méx vol.45 no.4 Ciudad de México oct./dic. 2001
Investigación
Contribution to the Biginelli Reaction, using a Bentonitic Clay as Catalyst and a Solventless Procedure
Manuel Salmón,1 Roberto Osnaya,2 Laura Gómez,2 Gabriel Arroyo,2 Francisco Delgado3 and René Miranda*2
1 Instituto de Química, Universidad Nacional Autónoma de México, Circuito Exterior, Ciudad Universitaria, Coyoacán, México 04510, D.F.
2 Departamento de Ciencias Químicas, Facultad de Estudios Superiores Cuautitlán, Universidad Nacional Autónoma de México, Av. Primero de Mayo s/n, Cuautitlán Izcalli, Estado de México 54740, México. E-mail: mirruv@latinmail.com
3 Departamento de Química Orgánica, Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, Prolongación Carpio y Plan de Ayala, Casco de Santo Tomás, México 11340, D.F.
Recibido el 19 de octubre del 2001.
Aceptado el 21 de diciembre del 2001.
This work is dedicated to Dr. Fernando Walls Armijo.
Abstract
The reaction of a set of five aldehydes, ethyl acetoacetate and urea or thiourea (Biginelli reaction) has been performed over a bentonitic clay as the catalyst, under solventless conditions using infrared irradiation as the energy source, obtaining the corresponding dihydropyrimidones .
Keywords: Biginelli esters, green chemistry, tonsil, bentonitic clay.
Resumen
Se realizó la reacción entre una serie de cinco aldehídos, con acetoacetato de etilo y urea o tiourea (reacción de Biginelli) empleando una arcilla bentonítica como catalizador en ausencia de disolvente y utilizando irradiación infrarroja como fuente de energía, obteniéndose las correspondientes dihidropirimidonas.
Palabras clave: Ésteres de Biginelli, tonsil, arcilla bentonítica.
Introduction
Some of the main objectives of green chemistry is to carry out reactions under solventless conditions with natural heterogeneous catalysts in order to be innocuous to the environment [1]. Complementary, it is worth mentioning that an ideal synthesis has been established as one in which a target molecule is produced in one step quantitatively, from available and inexpensive starting compounds, in an environmentally acceptable process [2].
Since the revision of fundamental synthetic reactions under heterogeneous catalysis represents the principal subject of continuos investigation of our research group [3], we now examine the Biginelli reaction with clay catalysis. The Biginelli reaction [4], described more than a hundred years ago and reviewed by Kappe [5], consists of the one-pot condensation of β-dicarbonyl compounds with aldehydes and ureas or thioureas affording dihydropyrimidine moieties (Scheme 1), some of them showing important pharmacological properties (i.e. calcium channel blockers, antihypertensive agents, alpha1-a-antagonists) [6]. The reaction is commonly performed in EtOH or THF under strong protic acid catalysis and combinations of Lewis acids with transition metal salts have been also used; in addition, a polyphosphate ester was recently employed improving the yield of the process [7].
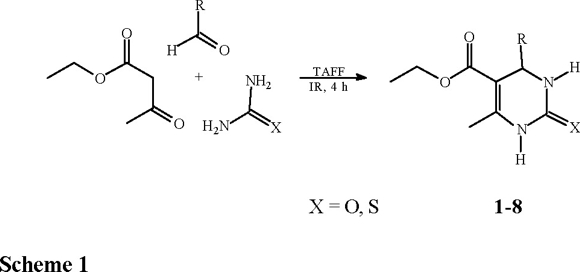
Related to our research program [8-9] on the use of TAFF, a commercial bentonitic clay [10] as a Lewis catalyst, we wish to notify that the aim of this paper is to report the corresponding results in order to obtain a set of dihydropyrimidones (DHPMs) (1-8) promoted by TAFF, under solventless conditions, using infrared irradiation as the energy source [11]. Moreover a contribution to green chemistry is offered since this new method is environmentally benign as well as economically feasible [10].
Discussion
In Table 1 have been summarized the experiments performed by this new method to prepare the DHPMs 1-8. These compounds were obtained with acceptable yields in an adequate reaction time [12].
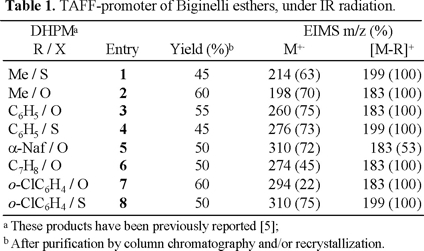
This novel technique offers a clean and easy method for the preparation of the target molecules. The reaction provided additional advantages such as an easy work-up and is carried out in absence of solvent. However, most reactions described in the Table showed no further progress after 4 h, as evidenced by TLC.
Our results also demonstrate that infrared irradiation can be used as a valuable means for activating organic compounds. To our knowledge, this is the first time that this energy source has been used for the promotion of this one-pot cyclocondensation. This environmental-friendly clay afforded a valuable alternative to promote a numerous efficient catalytic systems that have already been proposed for the achievement of DHPMs.
Experimental section
All aldehydes are commercially available (Aldrich Chemical Co.) and were employed without further purification. The reactions were monitored by TLC (n-hexane-AcOEt, 7:3) performed on percoated (0.25 mm) Merck silica-gel 60-F254 aluminum sheets, the product visualization was done using a 254 nm UV lamp. Melting points are uncorrected and were determined on a Fisher-Johns apparatus. The EIMS were performed on a JEOL JMS-SX 102 instrument.
General procedure for the preparation of 1-8. A mixture of aldehyde, urea and ethyl acetoacetate (8.226 mmol) was mixed with 500 mg of TAFF, and placed in a round-bottomed flask (50mL) equipped with a condenser, then it was irradiated by means of an infrared lamp and monitored by TLC during 4 h. After cooling, the product was extracted with Me2CO (20 mL) and the solvent evaporated under vacuum. The solid obtained was chromatographied (n-hexane-AcOEt, 7:3) and/or recristallyzed from EtOH, affording 1-8.
Acknowledgements
René Miranda and Gabriel Arroyo to DGAPA-UNAM: grant PAPIIT-IN215598 for financial support. Ms. Eva Hernández Godinez for technical asistance.
References and notes
1. Anastas P.T.; Williamson T.C. Green Chemistry, Frontiers in Benign Chemical Syntheses and Processes, Oxford University Press, 1998. [ Links ]
2. Wender, P.A.; Handy, S.L.; Wright, D.L. Chem. Ind. (London), 1997, 765-769. [ Links ]
3. Miranda, R.; Osnaya, R.; Garduño, R.; Delgado, F.; Álvarez, C.; Salmón, M. Synth. Commun. 2001, 31, 1587-1597, and refences therein. [ Links ]
4. Biginelli, P. Gazz. Chim. Ital., 1983, 23, 360-416. [ Links ]
5. a) Kappe, C.O. Tetrahedron, 1993, 49, 6937-6963 b) Kappe, [ Links ] C.O. Molecules, 1998, 3, 1-20. [ Links ]
6. Atwal, K.S.; Rovnyak, G.O.; O'Reilly, B.C.; Schwartz, J. J. Org. Chem., 1989, 54, 5898-5907 Kappe, [ Links ] C.O.; Fabian, W.M.F.; Semones, M.A. Tetrahedron, 1997, 53, 2803-2816. [ Links ]
7. a) Hu, E.H.; Sidler, D.R.; Dolling, U.-H. J. Org. Chem., 1998, 63, 3454-3457 b) Kappe, [ Links ] C.O.; Falsone, S.F. Synlett, 1998, 718-720. [ Links ]
8. Miranda, R.; Escobar, J.; Delgado, F.; Salmón, M.; Cabrera, A. J. Mol. Cat. 1999, 150, 299-305. [ Links ]
9. Obrador, E.; Castro, M.; Tamariz, J.; Zepeda, G.; Miranda, R.; Delgado, F. Synth. Commun. 1998, 28, 4649-4663. [ Links ]
10. Tonsil Actisil FF (TAFF), a comercial Mexican bentonitic clay, is easily available from Tonsil Mexicana S. A. de C. V. Mexico City, Mexico at US $1.30 / kg. Examined with X-ray fluorescence, this clay proved to have the following composition (in percent): SiO2, 74.5; Al2O3, 9.3; MgO, 0.4; Fe2O3, 1.3; CaO, 4.0; K2O, 0.4; TiO2, 0.4; H2O, 9.7. When X-ray thermodiffractograms were run, the laminar structure was found to be unstable above 150 °C. Quartz and cristobalite are also important components in the clay composition as observed by X-diffraction powder. The corresponding BET surface area was 198.718 m2g−1 and the pore volumen and average pore diameter were 32.04 × 10−2 cm3 g−1 and 77.8 Å, respectively. It is worth mentioning that a detailed characterization of the clay (29Si and 27Al MAS-NMR, SEM, IR-Py, DTA, and TG, Ho) is under review. Miranda, R.; Ríos, H.; Salmón, M.; Cogordán, J.A.; Castro, M; Delgado, F. J. Appl. Cat. 2001. [ Links ]
11. Alcerreca, G.; Sanabria, R.; Miranda, R.; Arroyo, G.; Tamariz, J; Delgado, F. Synth. Commun. 2000, 30, 1295-1301. [ Links ]
12. The products 1-8, were identified by physical and spectral correlation with literature reports (mp, PNMR and EIMS): for example, all the molecular ions (Table) are in agreement with the structure of a Biginelli ester as well as the very intensive fragment [M-R]+.














