Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Sociedad Química de México
versión impresa ISSN 0583-7693
Rev. Soc. Quím. Méx vol.45 no.2 Ciudad de México abr./jun. 2001
Investigación
Lignin degradation products from corn stalks enhance notably the radial growth of basidiomycete mushroom mycelia
Miguel J. Beltrán-García,1,2 Aideé Orozco,1 Ivan Samayoa1,3 and Tetsuya Ogura*1
1 Departamento de Química, Universidad Autónoma de Guadalajara, A.P. 1-440, Av. Patria No. 1201, Lomas del Valle, Guadalajara 44100, Jal., México. Tel: 3-610-1010, ext. 2433, Fax: 3-610-1610. E-mail: togura@uagunix.gdl.uag.mx
2 Departamento de Bioquímica, Instituto de Fisiología Celular, UNAM, México 04510, D.F.
3 Base de Frutas de México, SA de CV. Tlajomulco, Jalisco, México.
Recibido el 4 de diciembre del 2000.
Aceptado el 2 de julio del 2001.
Abstract
Milled corn stalk (crude) was cleaned by diverse solvents (cleaned). When 1000 ppm of cleaned was added to Peptone / Glucose / Agar (PGA), the growth rate of Pleurotus ostreatus and Pleurotus sajor-caju was doubled. The mycelial diameter also increased by a supplementation of a hot water extract of cleaned corresponding to 1000 ppm, which was composed of 400 µg / g-stalk of nonvolatile compounds including 60 µg / g-stalk of phenolic monomers. An IR spectrum of the extract was very similar to that of cleaned and commercial lignin. Supplementation of crude or cleaned to Malt extract / Sucrose / Agar (MSA) promoted mycelial growth by 30 % in all five basidiomycete studied. A commercially available Kraft lignin supplemented to MSA showed cleaned-like growth promotions. The hot water extract showed growth promotion to the mycelia at a range of 10−6−10−7 M as seen in plant hormones. Therefore, we suggest these fungi use the enzymatic degradation products of lignin as a signal to faster mode of growth. This must be an important tactic of the fungi in securing their niche by degrading a vegetal biomass before competitors encroach upon it.
Keywords: Basidiomycetes, corn stalk; growth promotion, lignin-derived phenols, Pleurotus.
Resumen
Tallos de maíz secos y molidos (crude) fueron lavados por varios solventes (cleaned). Cuando se añadió 1000 ppm de tallos lavados al medio de cultivo Peptona / Glucosa/ Agar (PGA), el crecimiento de Pleurotus ostreatus y Pleurotus sajor-caju se incrementó al doble. El crecimiento del micelio también aumenta cuando se añaden 1000 ppm de un extracto acuoso de tallos lavados (cleaned) compuesto de 400 µg de sustancias no volátiles por gramo de tallo. Este extracto contenía 60 µg / g de tallo de monómeros fenólicos. El análisis por IR muestra que el espectro del extracto es similar al de cleaned así como al de la lignina comercial. El crecimiento del micelio de 5 basidiomicetos se promovió en un 30 % al añadir crude y cleaned al medio de cultivo. El incremento en el crecimiento también ocurrió cuando se adicionó la lignina comercial al medio de cultivo de Agar Extracto de Malta. Este incremento fue similar al inducido por cleaned. El extracto acuoso incrementó el crecimiento en un intervalo de concentración de 10−6 a 10−7 M, tal y como se observa en las plantas cuando se les añade hormonas. Sugerimos que estos hongos basidiomicetos usan a los productos de la degradación enzimática de la lignina como una señal para acelerar su crecimiento. Este hecho puede ser una táctica importante de los hongos para degradar la biomasa vegetal antes que sus posibles competidores.
Palabras clave: Basidiomicetos, fenoles derivados de la lignina, Pleurotus, promoción del crecimiento, tallos de maíz.
Introduction
Corn stalks remaining in the field after harvest contain 43 % polysaccharide consisting mainly of hemi cellulose and cellulose, 29 % lignin, 7 % proteins, 5 % ash, and 16 % others [1]. A part of this renewable lignocellulosic biomass has been used for ruminant food and cultivation of edible mushrooms.
During the cultivation of various basidiomycetes, we observed that a small amount of corn stalk chips added to a culture medium accelerated the growth rate. We now supplement routinely with 1 g corn stalk powder for a 1 l culture medium for fungi culture. Various publications describe fungal growth promotion by plant constituents. It has been shown that Pleurotus ostreatus grows faster on cotton stalks than on wheat straw, and that water extracts of cotton stalks contain compounds that increase fungal growth rate by 50 % in liquid and solid culture [2]. After separation by thin-layer chromatography of the cotton extracts, the biologically active fraction showed flavone-like characteristics. When a cotton extract was added to wheat straw, degradation was increased significantly. Ardon et al. [3] showed that cotton stalk extract enhanced laccase activity of P. ostreatus.
Inaba et al. in 1981 and Kawamura et al. [4-5] found that the growth rate of basidiomycetes such as P. ostreatus and Lentinula edodes were promoted by adding lignin to a peptone-glucose medium. In relation with growth promotion of fungal mycelia some authors showed that phenolic compounds derived from lignin promoted the growth of the genus Lentinula [6-8].
In spite of a wealthy of publications, the scientific communities have not been convinced enough of the effectiveness of lignocellulosic materials. We report in this paper that degradation products of lignocellulosic materials are responsible to the growth promotion of fungal mycelia. The degradation products, including phenolic compounds were effective to promote growth rates at a concentration of µM order, which is comparable to the effective concentrations seen in plant hormones.
Materials and methods
Fungi cultures. Agaricus bisporus ATCC 36415 (Abi), Agaricus campestris ATCC 2334 (Aca), Lentinula edodes ATCC 48860 (Led), Pleurotus ostreatus ATCC 38540 (Pos), and P. sajor-caju ATCC 32078 (Psc) were purchased from the American Type Culture Collection (ATCC, 12301 Rockville, MD 20852). All the fungal mycelia were grown on Malt extract / Sucrose / Agar (10:40:20, g / l) (MSA) at ambient temperature (25 ± 2 °C). Agar discs 0.7 cm in diameter were removed from the periphery of actively-growing Petri dish cultures and placed in the center of Petri dishes (90 mm × 15 mm) containing MSA (20 mL) with or without supplementation. The growing rates were measured by averaging two perpendicular mycelial growth diameters substracting 0.7 cm of the initial disc diameter. All experiments were triplicated.
In another series, Pos and Psc mycelia were grown on a Peptone / Glucose / Agar (10:40:20, g / l) (PGA) broth. The mycelial growths were monitored by similar manner to the experiments using MSA.
Plant materials and cleaning procedures. Corn was seeded and harvested by Francisco Velez at La Resolana Ranch, Acatic, Jalisco, Mexico. No pesticide was applied. The corn stalk was harvested when corn ears matured and dried in the shade. It was kept at least one year in a well-ventilated dark and cool place, which was ground and collected a range of size of 16-200 mesh (crude).
Forty-five grams of crude were extracted using a Soxhlet's extractor employing 350 mL of toluene for 2 h at which time the toluene extract was colorless. The powder was dried in air for 24 h. There followed successive Soxhlet-extractions by chloroform, ethanol and water using similar procedures. The powdery product was refluxed by a mixture of 100 ml of benzene and 100 ml of ethanol for 2.5 h. Finally the product was refluxed further by 100 ml of ethanol for 2.5 h. The dried product is called cleaned.
Hot water extraction of cleaned. Ten grams of cleaned suspended in 150 mL of water placed in an Erlenmeyer flask (500 mL) was heated to 120 °C for 1 h in an autoclave similar to a routine sterilizing procedure. The solution was separated by filtration and dried using a rotary evaporator. The residue was dried further by a nitrogen flow for 12 h at room temperature and 3.96 mg of a coffee-brown creamy matter was obtained. IR spectra of the residue approximated very closely that of commercial lignin mixture (Sigma Chemical) (3440 (st), 2910, 1600, 1320-1420, 1080-1150 (st) and 600 cm−1) and of cleaned.
An ethyl ether extract of the residue (20 mL × 3 times) was dried, followed by dissolution in 500 µL of methanol for analysis by gas chromatography using a Varian Model 3300 equipped with a flame ionization detector employing a column AT-1 (Alltech), 30 m × 0.53 mm × 1.2 µm. Conditions: Column temperature 70 °C to 270 °C at 10 °C / min; injector 150 °C; detector, FID at 300 °C.
Results
P. ostreatus and P. sajor-caju were grown on PGA, which does not contain plant materials. Surprisingly the supplementation of PGA with cleaned doubled their growth rate as seen in figures 1 (Ͱ) and 2 (Ͱ) compared to the growth rate in none supplemented PGA (u). cleaned was heated in water at 120 °C for 1 h as the pasteurization of culture media. PGA supplemented by the resultant aqueous solution promoted the mycelial growth as seen in figures 1 (*) and 2 (*) but considerably less than cleaned.
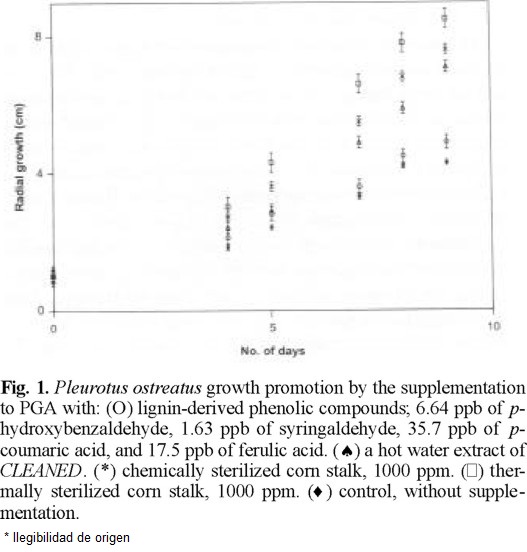
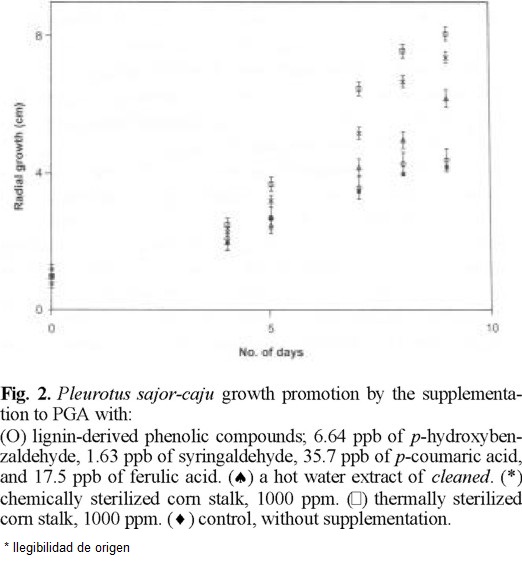
One g of cleaned yielded 396 µg of nonvolatile compounds including p-coumaric acid (35.9 µg), ferulic acid (17.5 µg), p-hydroxybenzaldehyde (6.64 µg), and syringaldehyde (1.63 µg) as phenolic monomers on heating in water at 120 °C for 1 h. IR spectra of the residue of evaporation was very similar to that of cleaned and of commercial lignin. Hence, the mycelia were grown on PGA supplemented by a phenolic mixture of the same composition. Only a slight increase of growth was observed under this condition as shown figures 1 (O) and 2 (O).
Cleaned sterilized by an equivolume mixture of ethanol and benzene was added to a previously pasteurized PGA. The mycelia grew almost double as seen in figures 1 (*) and 2 (*) compared to the control.
Since the basidiomycetes grew nearly linearly during 3rd to 9th days as seen in figures 1 and 2, all experimental data on mycelial diameters during this stage were calculated by linear least square treatments and they are summarized in Table 1. The growing velocities based on the slopes reached to 2.0-2.3 times in chemically sterilized fiber and 2.2-2.7 times in thermally sterilized ones compared to the controls. The mixture of phenolic compounds also accelerated, 40 % for Pos and 3 % for Psc The statistical confidence ranges evaluated comparing linear regression equations of slopes between the phenolic monomers supplementation and control remain 0.99 > P for Pos and Psc [9]. The differences between the controls and the PGA supplemented with hot water extract, chemically or thermally sterilized fiber were highly trustworthy. Nevertheless, the standard deviations seen from Table 1 are considerably large. Every run deviates little from a curve; however, deviations within runs were considerably large. We found that mycelial growth is slowed notably by a slight increase of light, hence it has been concluded that the control of light intensity was not satisfactory in our experiments. All data obtained from 278 runs for Pos and 278 runs for Psc are shown in Table 1 and figures 1 and 2 in order to demonstrate the relative effects to the growth rate but not the absolute growth velocities.
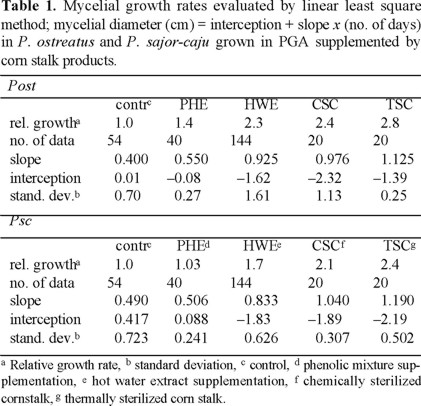
Mycelia of Abi, Aca, Led, Pos and Psc were grown on MSA supplemented by these products at a concentration range of 1-1000 ppm. Apparently both of crude and cleaned promoted all mycelial growth in similar extent by a concentration dependent manner. However, the growth rate increased by only less than 30 % (data not shown).
Supplementation of a commercially available Kraft lignin to MSA showed slight growth promotions of five basiomycete mycelia at a low concentrations ranging to 1-10 ppm, however growth inhibition suppressed the promotion at higher concentrations.
Discussion
It is well documented that fungal mycelial growth is accelerated by phenolic compounds [6-12]. Cai et al. and Buswell and Erikson [13-14] studied diverse lignin-derived phenolic compounds and their methylated derivatives at the concentration range of 1-10 mM on the growth of edible mushrooms such as L. edodes and P. sajor-caju. They found slight promotions and inhibitions in some phenolic compounds at low concentrations. However, a phenolic concentration less than 1 mM is not available in these studies. On the other hand, the concentrations of four phenolic compounds summed up to only 3.7 µM in our supplementation. Yet it showed notable growth accelerations.
Plant growth hormones are the compounds that induce plant growth at lowest concentration ever known, regardless that each one acts in different manner from growth promotion to acceleration of senescence. Thus, the most common plant hormones such as gibberellic acids [15] and indole acetic acid [16] enhance plant growths as low concentration range as 10−6 to 10−8 M. Accordingly the phenolic concentration, 3.7 µM, is comparable to the plant hormone action.
The hot water extract of cleaned showed a greater growth acceleration than the phenolic mixture. The major part of the extract, approximately (330 µg) / (g-cleaned), consists in lignin-like compounds composing of phenolic unit. Assuming that the extract is a mixture of phenolic oligomers with an average molecular weight 1000, a supplementation of 1 g-cleaned per l l-MSA results in 3.3 × 10−7 M of the compounds. Lin and Lebo [17] described that the phenolic monomers were liberated by thermal hydrolysis of grass lignin; hence, the formation of oligomers as well as the monomers must be reasonable.
These mycelia grew well faster than the hot water extract when PGA was supplemented with solid cleaned sterilized even chemically or thermally. However, the later grew slightly faster than the former, showing the chemically sterilized cleaned was degraded by fungal enzymes alone but the thermally sterilized one summed the effects of the thermal and enzymatic degradation products.
Azuma and Tetsuo described a preparation method of lignin-carbohydrate complexes [18]. In the first step, they obtained a product composing of lignin-carbohydrate complexes and pectin employing a similar procedure to prepare the cleaned, followed by a removal of pectin to get the complexes. Pectin supplemented to PGA up to 1000 ppm showed no growth promotion to Pos and Psc. On the other hand, these fungi were promoted notably by 4 µM of lignin-derived phenolic monomer compounds and much more by cleaned. Therefore, lignin solubilized by degradation such as lignin-carbohydrate complexes, phenolic oligomers and monomers might be responsible to the growth promotion of the fungal mycelia. Really, Inaba et al. in 1982 [19] found that sulfonated lignin-carbohydrate complexes added 1 % to culture media promoted basidiomycetes growth.
Culture media based on potato infusion and grain hydrolysates such as malt and Aspergillus oryzae-grown rice are used frequently to culture basidiomycete mycelia, indicating the importance of the fragmented lignin of the plant origin in these media. On the contrary, culture media that do not contain plant constituents such as PGA might be employed for fungi, which do not degrade lignin. The effectiveness of MSA over PGA was tested using A. bisporus, P. ostreatus, and P. sajor-caju as shown in Table 2. They grew much faster on MSA than on PGA, in accord with the common preference to use MSA for these fungi.
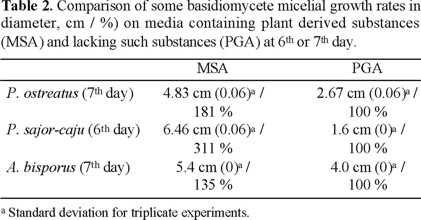
Interestingly, the PGA supplemented with 1000 ppm of cleaned potentiated the mycelial growth velocities of P. ostreatus and P. sajor-caju almost as effective as MSA as seen from Table 1: P. ostreatus grew even faster and P. sajor-caju a little slower. The similarity between MSA and cleaned-supplemented PGA indicates that lignin derivatives play a role to accelerate the fungal growth.
Basidiomycetes actively degrade lignin in biomass using extracellular enzymes, including fungal peroxidases [20] and laccase [21]. Let us suppose that a growing P. ostreatus mycelium germinated from a spore meets a log. Then, the fungal enzymes degrade lignin and the degradation products including phenolic monomers diffuse getting behind of the mycelium at the front end accelerating mycelial growth. As a result, more degradation takes place. This must be an important tactic of the fungi in securing their niche in a biomass before competitors encroach upon it.
The enzymatic degradation products of lignin might switch the signal controlling growth rate to a faster mode. However, there are enormous numbers of chemical species as the product such as phenolic compounds, oligomers with different degrees of oligomerization derived from phenolic compounds as the building block, and lignin-carbohydrate complexes with a variety of chain length and monomer components. It must be difficult that receptors respond directly to all the products.
Phenolic compounds are oxidized to catechol (o-dihydroxybenzene) or hydroquinone (p-dihydroxybenzene) derivatives in the presence of fungal dioxidase or laccase. These dihydroxybenzene derivatives are easily oxidized further to quinone derivatives enzymatically or nonenzymatically. The oxidation produces two short-lived HO2 (hydrogen superoxide) molecules, which disproportionate to H2O2 and O2 enzymatically by superoxide dismutase (SOD) or nonenzymatically [22]. Consequently, these fungi may detect HO2 or H2O2 in stead of the lignin degradation products.
It is widely accepted that ingestion of dietary fiber is indispensable to maintain human and animal health. Nevertheless, a fully acceptable reasoning has not been proposed yet. On the other hand, 20µL of an Escherichia coli (ATCC 25922) culture at a log phase culture (1500 × 106 cells / mL ) were inoculated to peptone-glucose broth and the growth was monitored by turbidity with an spectrophotometer at 450 nm. The results obtained showed that the growth was enhanced by 40 % by a supplementation with the hot water extract of cleaned corresponding to 1 g of cleaned to 1 l of culture media (data not shown). However, the behavior of intestinal microflora is complicated, especially the lignin hydrolyzing microorganisms and the ones promoted by the hydrolysates are not necessarily the same. Moreover, the beneficial microorganisms must be promoted, whereas the malignant ones must be inhibited in order to contribute to the health.
Acknowledgments
We thanks Prof. Dr. Takeo Tabata (Kobe Women's University Seto Junior College) for his stimulating and valuable discussions. MJB-G thanks to Consejo Nacional de Ciencia y Tecnología for the scholarship #93376, the Government of the State of Veracruz, and Fundación Telmex for the scholarship.
References
1. Chahal, D.S.; Hachey, J.M. In: Agricultural and synthetic polymers: Biodegradability and utilization. Glass, J.E.; Swift, G., Eds., American Chemical Society Symposium Series 1990, American Chemical Society, Washington D. C., 1990, 306-310. [ Links ]
2. Platt, M.W.; Hadar, Y.; Henis, Y.; Chet, I. Eur. J. Appl. Microbiol. Biotechnol. 1983, 17, 140-142. [ Links ]
3. Ardon, O.; Kerem, Z.; Hadar, Y. Can. J. Microbiol. 1998, 44, 676-680. [ Links ]
4. Inaba, N.; Iizuka, Y.; Koshijima, T. Mokuzai Gakkaishi 1981, 27, 231-236. [ Links ]
5. Kawamura, N.; Goto, M.; Nakamura, Y. Trans. Mycol. Soc. Jpn. 1983, 24, 213-222. [ Links ]
6. Ikegaya, N.; Goto, N. Trans. Mycol. Soc. Jpn. 1988, 29, 401-11. [ Links ]
7. Inaba, N.; Iizuka, Y.; Koshijima, T. Mokuzai Gakkaishi 1980, 26, 482-487. [ Links ]
8. Shuen, S.K; Bushwell, J.A. Lett. App. Microbiol. 1992, 15, 12-14. [ Links ]
9. Zar, J.H. Biostatistical analysis. 3rd ed., Prentice Hall, New Jersey, 1996, 353-369. [ Links ]
10. Wat, C. K.; Towes, G. H. N. Phytochemistry 1975, 14, 663-666. [ Links ]
11. Gupta J. K.; Humps S. G.; Bushwell J. A.; Eriksson K. E. Arch. Microbiol. 1981, 128, 349-354. [ Links ]
12. Lavee, S.; Harshemesh, H.; Avidan, N. Acta Horticulture 1986, 179, 317-328. [ Links ]
13. Cai, Y.J.; Buswell, J.A.; Chang, S. T. World J. Microbiol. Biotechnol. 1993, 9, 503-507. [ Links ]
14. Buswell, J.A.; Erikson, K.-E.L. World J. Microbiol. Biotechnol. 1994, 10, 169-174. [ Links ]
15. Libbenga, K. R.; Mennes, A. M. In: Plant Hormones and Their Role in Plant Growth and Development, Davis, P.J. Ed. Kluwer Academic Publishers, Dordrecht, Netherland, 1990, 272-297. [ Links ]
16. Cleland, R. E. In: Plant Hormones and Their Role in Plant Growth and Development, Davis, P.J. Ed.. Kluwer Academic Publishers , Dordrecht, Netherland. 1990, 132-147. [ Links ]
17. Lin, S.Y.; Lebo, Jr. S.E. In: Encyclopedia of Chemical Technology, Vol. 15, John Wiley and Sons, 1995, 268-289. [ Links ]
18. Azuma, J.; Tetsuo, K. Methods Enzymol. 1988, 161, 12-18. [ Links ]
19. Inaba, N.; Iizuka, Y.; Koshijima, T. Mokuzai Gakkaishi 1982, 28, 319-324. [ Links ]
20. Babitskaya, V. G.; Shcherba, V.V. Microbiology 1999, 68, 58-62. [ Links ]
21. Muñoz, C.; Guillen, F.; Martínez, A. T.; Martínez, M. J. Current Microbiol. 1994, 34, 1-5. [ Links ]
22. Leonowicz, A.L.; Maniszewska, A.; Luterek, J.; Ziegenhagen, D.; Wojtas-Wasilewska, M.; Cho, N-S.; Hofrichrer, M. Fungal Gen. Biol. 1999, 27, 173-185. [ Links ]














