Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Sociedad Química de México
versión impresa ISSN 0583-7693
Rev. Soc. Quím. Méx vol.45 no.1 Ciudad de México ene./mar. 2001
Investigación
Adsorptive stripping voltammetry of In(III) in the presence of pyrogallol red in chloride-acetate media
J. C. Aguilar, E. Rodríguez de San Miguel and J. de Gyves*
Departamento de Química Analítica, División de Estudios de Posgrado, Facultad de Química, UNAM. Ciudad Universitaria, México 04510, D.F.
Recibido el 9 de octubre del 2000.
Aceptado el 15 de marzo del 2001.
Abstract
In this work optimal working conditions were established for the adsorptive stripping analysis of In(III) on a hanging mercury drop electrode with pyrogallol red as the preconcentration agent in chloride-acetate media using staircase and square-wave voltammetry (SCV and SWV). The influence of ligand adsorption and concentration, supporting electrolyte and pH on the voltammetric response was studied. From these results the stoichiometric ratio of the complex and the value of its corresponding formation constant were determined. Under optimized chemical and instrumental conditions limits of detection (3σ) of 7.0 × 10−9 M and 4.0 × 10−9 M were obtained for SCV and SWV, respectively, using a deposition time of 90 s. The relative standard deviations (n = 21) were 3.3 and 4.8 % for a 1.5 × 10−7 M In(III) concentration. The results of an analysis carried out for indium present in aluminum alloys were in good agreement with those obtained using ICP-AES as an alternative method.
Keywords: Adsorptive stripping voltammetry, In(III), pyrogallol red.
Resumen
En este trabajo se establecen condiciones óptimas para el análisis de In(III) por voltamperometría (SCV y SWV) de redisolución con preconcentración adsortiva, usando un electrodo de gota colgante de mercurio y rojo de pirogalol como agente de preconcentración en un medio de cloruro-acetato. Se estudió la influencia de la adsorción y la concentración del ligando, del electrolito soporte y del pH en la respuesta voltamperométrica. A partir de los resultados obtenidos se determinaron la relación estequiométrica del complejo y el valor de la constante de formación. La evaluación de los métodos electroquímicos indica que el In(III) puede cuantificarse con buena sensibilidad, precisión y exactitud. Los límites de detección (3σ) usando un tiempo de depósito de 90 s son 7.0 × 10−9 M y 4.0 × 10−9 M para SCV y SWV, respectivamente. Las desviaciones estándares relativas (n = 21) fueron de 3.3 y 4.8 % para una concentración de [In(III)] = 2 × 10−7 M. Los resultados del análisis de indio, presente en una muestra de una aleación a base de aluminio, realizado de acuerdo con el método electroquímico propuesto se ajustan bien a los obtenidos mediante ICP-AES.
Palabras clave: Voltamperometría de redisolución adsortiva, In(III), rojo de pirogalol.
Introduction
Adsorptive stripping voltammetry is a powerful tool for quantitative analytical purposes. Several methods have been developed involving metal ion complexation with organic compounds showing surface activity, followed by an adsorptive preconcentration step and by the use of a voltammetric technique such as linear scan (LSV) [1], differential pulse (DPV) [2], staircase (SCV) [3] and square-wave voltammetry (SWV) [4]. Pulse voltammetric techniques offer greater sensitivities than LSV and are preferred because of their better discrimination of capacitive currents. Particularly, SWV allows faster scan rates than DPV [5], making it a widely used technique. It has also been shown that adsorption of reactants and products of redox reactions may cause an enhancement of the SWV response due to the absence of a limiting mass transfer step, especially in cases where the redox reactions are quasi-reversible [6].
In(III) can be reversibly reduced at a mercury electrode in the presence of polarizable ligands such as chloride, bromide, iodide and acetate [7], but its electrochemical response in diffusion-controlled regime conditions can only be recorded for concentrations well above 10−7 M [8]. Adsorptive preconcentration of In(III) can be achieved using different organic ligands such as cupferron and oxine [9], allowing indium determination down to 10−9 M. Other organic compounds can be employed as well. In the present work the voltammetric behavior of In(III) is studied in a chloride-acetate medium in the presence of pyrogallol red (PR), which has been previously used as a complex-forming agent for adsorptive stripping voltammetry [10, 11].
Experimental
Apparatus and reagents
The voltammetric measurements were performed using a EG&G Princeton Applied Research (PAR) model 273 potentiostat / galvanostat controlled by a Hyundai 486 PC through the PAR model 270 electrochemical software, in conjunction with a PAR model 303 static mercury drop electrode (SMDE) set to hanging mercury drop electrode (HMDE) mode with the large drop size selected (electrode area: 2.5 × 10−6 m2). All potential values reported herein are given as referred to the Ag/AgCl (satd. KCl) electrode. Stirring of solutions in the cell was accomplished using a PAR model 305 magnetic stirrer.
All reagents were analytical grade and used as supplied by Aldrich. Indium standard solutions were prepared from InCl3 and from a 0.990 g dm−3 Aldrich atomic absorption standard solution. Proper dilutions of stock solutions were made with deionized water (resistivity: 0.17 MΩ m) to obtain the working solutions.
Procedure
All measurements were made at room temperature (23 ± 1 °C) and after passing a stream of nitrogen through a 10 mL cell during 4 minutes. The best working conditions were established from the dependence of peak currents on pH, chloride ion, acetate buffer and PR concentrations, and deposition time and potential (pH = 4.0, pCl = 2.00, pAcO = 1.92, [PR] = 1.5 × 10−6 M, td = 90 s and Ed = −0.30 V). Analysis of indium content in two samples of aluminum-zinc alloys by means of a standard addition method was performed after dissolving 0.500 g of the material in 8 mL of concentrated hydrochloric acid and transferring the solution to a 100 mL volumetric flask.
The cells employed were soaked overnight in dilute nitric acid (20 % v/v) before every experiment in order to avoid analyte adsorption on the cell walls.
Results and discussion
PR (Fig. 1) is an organic compound able to form complexes with In(III), just as one of its derivatives (bromopyrogallol red [12]) does, because of its hard base character. The highly conjugated structure of these compounds allows to consider them as potential preconcentration agents for indium adsorptive stripping voltammetry. The adsorption of PR on a mercury electrode is shown in figures 2 and 3.


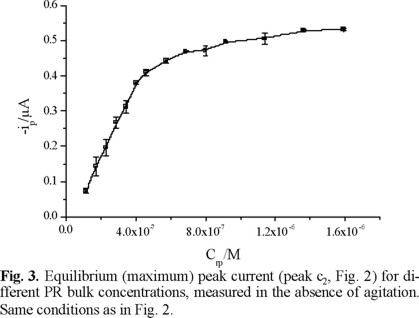
The signal c2 that is observed after addition of the ligand to the supporting electrolyte in figure 2, is due to the irreversible reduction of the pseudoquinonic group. This electrochemical response of PR allows to detect the changes of its surface concentration, as seen in figure 3, where equilibrium peak current of PR reduction (obtained in the absence of convection) is shown as a function of PR bulk concentration. PR surface concentration follows a linear relation with bulk concentration, as predicted by Koryta's equation [13] over the range of 1 × 10−7-4 × 10−7 M. At higher bulk concentrations, a limiting value is reached for the peak current of PR reduction, due to the fact that the maximum surface concentration has been attained.
Both reduction and oxidation peaks (ip = 12 nA) for 5 × 10−7 M In(III) in a 0.010 M chloride-0.012 M acetate (pH = 4) were observed at −0.55 and −0.53 V [10], respectively. Under the conditions given in figure 2, In(III) reduction takes place at −0.59 V (peak c1, Fig. 2) and the peak current increases dramatically (750 nA for 2 × 10−7 M In(III)).
It was pointed out in the introduction that the presence of chloride and acetate ions facilitates In(III) electrochemical reduction, so the best composition of the medium was investigated by varying acetate buffer and chloride concentrations, and the pH value of the solutions. The dependence of the peak current for indium and PR reduction on buffer concentration (total acetate concentration) is presented in figure 4. As the acetate concentration in solution increases, peak c2 shows a slightly growing tendency, while peak c1 reaches a maximum value between buffer concentrations of 2 × 10−3 and 3.2 × 10−2 M.

The same behavior is observed for the dependence of both reduction peak currents on chloride ion concentration (Fig. 5). The different effects of these parameters on the electrochemical response of the ligand and the complex can be explained on the basis of the formation of indium complexes with acetate and chloride ions. Once the anion concentration is high enough, the fraction of total indium concentration in the form of the In(III)-PR complex diminishes gradually because of the metal ion masking.

The pH value of the solution is also a critical parameter to be considered. The best pH for indium analysis was found to be a value of 4. Between pH values of 3.5 and 4.5, the In(III)-PR reduction peak (c1) reaches a maximum (Fig. 6). The PR reduction peak (c2) increases with pH, due to deprotonation of PR, and the presence of a larger negative charge on the molecule promotes its adsorption on the mercury electrode. At low pH values, PR is totally protonated and its surface and complexing activities are also lowered. On the other side, indium forms hydroxo compounds which may interfere with PR at high (> 5) pH values.
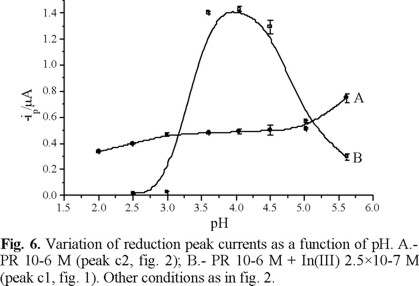
With all this information, the best supporting electrolyte composition for quantitative analysis of indium was established to be: Cbuffer = 0.012 M; CCl = 0.010 M, CPR = 1 × 10−6 M and pH = 4.0.
It is possible to estimate the value of the stability constant of the In(III)-PR complex using the following equation [14]:
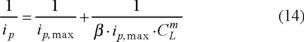
where ip is the measured peak current, ip, max is the value observed for the peak current when all the metal is complexed, CL is the concentration of the ligand and m is the number of ligand molecules attached to the metal ion. Since a straight line is obtained when plotting ip−1 as a function of CL−1, it is assumed that m = 1. From this analysis a 1:1 composition for the electroactive complex was determined, and the stability constant has a log value of 6.2 ± 0.2 under the optimum working conditions.
In this medium, peak current c1 is a linear function of indium concentration when an excess of PR is present in the solution. The linearity range under these conditions goes from 10−8 to 5 × 10−77 M of In(III) when using SCV as electrochemical technique and a td = 90 s, and the sensitivity and detection limit (3σ) of the method are then 2.72 A L mol−1 (σslope 0.43 × 10−1 A L mol−1) and 7 × 10−9 M, respectively. Both sensitivity and detection limit can be improved by employing different electrochemical techniques and deposition times. The use of SWV renders a 6-fold increment in the sensitivity (18.52 A mol−1, σslope 0.29 A L mol−1) when using a td = 90 s and a detection limit of 4 × 10−9 M.
In order to test the procedure of indium analysis, indium content in two samples of aluminum-base alloys was determined. It was found that aluminum interferes with indium determination, but it was possible to overcome this problem by adding sodium fluoride to the supporting electrolyte. A 4-fold excess of NaF with respect to Al(III) calculated concentration suffices to mask Al(III) present in the supporting electrolyte after sample dissolution without interfering in the In(III)-PR complex formation. These analyses were compared with ICP-AES measurements made for the same samples. Table 1 resumes the results obtained.

Conclusions
PR forms a 1:1 complex with In(III) in the chloride-acetate medium that adsorbs on a mercury electrode. These properties allow to use PR as a preconcentration agent for indium ultra-trace analysis by a SWV electrochemical method. The best working conditions achieved allowed to analyze the indium content in aluminum-base alloys. The accuracy of the electro-analytical method was established by comparison with an ICP-AES. Matrix effects (interference due to aluminum) were eliminated by additions of sodium fluoride.
Acknowledgements
The authors would like to thank the financial supporting from CEE project CI1*/0522 MEX(JR) and from DGAPA (Dirección de Asuntos del Personal Académico, UNAM) project IN303589.
References
1. a) Van den Berg, C. M. G.; Huang, Z. Q. Anal. Chim. Acta, 1984, 164, 209-222. [ Links ] b) Bastos, M. B. R.; Moreira, J. C.; Farias, P. A. M. Anal. Chim. Acta, 2000, 408, 83-88. [ Links ]
2. a) Palaniappan, R.; Kumar, T.A. Analyst, 1993, 118, 293-296. [ Links ] b) Bond, A. M.; Kratsis, S.; Newman, M. G. Anal. Chim. Acta, 1998, 372, 307-314. [ Links ]
3. Economou, A.; Fielden, P. R.; Packham, A. J. Analyst, 1994, 119, 279-285. [ Links ]
4. a) Economou, A.; Fielden, P. R. Analyst, 1993, 118, 47-51. [ Links ] b) El-Maali, N.A.; El-Hady, N.A. Anal. Chim. Acta, 1998, 370, 239-249. [ Links ]
5. Osteryoung, J.; O'Dea, J. J., in: Electroanalytical Chemistry, Vol. 14, Bard, A.J., Ed., Marcel Dekker, New York, 1986, 209-308. [ Links ]
6. Komorsky-Lovric, S.; Lovric, M. Fresenius Z. Anal. Chem., 1989, 335, 289-294. [ Links ]
7. Losev, V.V.; Molodov, A.I., in: Encyclopedia of electrochemistry of the elements, Ed. Nagaosa, Y. Talanta, 1979, 26, 987-990. [ Links ]
9. Sun, C.; Wang, J.; Ho, W.; Jin, W. J. Electroanal. Chem. 1991, 306, 251-258. [ Links ]
10. Aguilar, J.C., Master in Science Thesis "Contribuciones al estudio electroanalítico del sistema In(III)-rojo de pirogalol", 1996, UNAM, México, D.F. [ Links ]
11. Safavi, S.; Shams E. [ Links ] Anal. Chim. Acta, 1999, 385, 265-272.
12. Jadhav, S.G.; Murugaiyan, P.; Venkateswarlu, C. Anal. Chim. Acta, 1976, 82, 391-399. [ Links ]
13. Laviron, E. J. Electroanal. Chem., 1974, 52, 355-393. [ Links ]
14. Zhao, J.; Jin, W. J. Electroanal. Chem., 1989, 267, 271-278. [ Links ]














