Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista de la Sociedad Química de México
versão impressa ISSN 0583-7693
Rev. Soc. Quím. Méx vol.44 no.3 Ciudad de México Jul./Set. 2000
Investigación
Growth of CuO films on fibreglass by CVD process
Jorge Ramírez-Ortiz,1* Tetsuya Ogura,2 Jorge Medina-Valtierra,3 Sofía E. Acosta-Ortiz,3 Pedro Bosch,4 J. Antonio de los Reyes,4 y Víctor H. Lara4
1 Facultad Ciencias Químicas, Universidad Autónoma de Zacatecas, Carr. Cd. Cuauhtémoc Km 0.5, Guadalupe 98600, Zacatecas. Fax: 52 (4) 9230137. E-mail: jramirez@cantera.reduaz.mx
2 Departamento. de Química, ICET, Universidad Autónoma de Guadalajara. Av. Patria 1201, Guadalajara 44100, Jalisco.
3 Departamento. de Ingeniería Química, Instituto Tecnológico de Aguascalientes. Av. Adolfo. López Mateos 1801 Ote. Aguascalientes 20256, Aguascalientes.
4 Universidad Autónoma Metropolitana-Iztapalapa, Av. Michoacán y La Purísima s/n, Iztapalapa, México 09340, D.F.
Recibido el 19 de junio del 2000.
Aceptado el 4 de septiembre del 2000.
Abstract
To develop a novel material, constituted by cupric oxide (CuO) films deposited on fibreglass, we have used the chemical vapour deposition (CVD) of 2,4-pentanedionate copper (II), Cu(acac)2 as a precursor, and fibreglass as substrate. The resulting fibreglass with a film of CuO can be used either as a conducting fibber or an oxidation catalyst. Over the fibreglass, a polycrystalline coating was observed, conformed by a continuous arrangement of 0.2-0.4 µm aggregates of CuO.
Key words: CVD, CuO deposition, fibreglass, characterisation.
Resumen
Para desarrollar un material novedoso, constituido de fibra de vidrio recubierta con una película de óxido de cobre(II) CuO, utilizamos la técnica de deposición química de vapor CVD, como precursor bis(acetilacetonato)cobre(II) Cu(acac)2 y como sustrato la fibra de vidrio. La fibra de vidrio recubierta con una película de CuO puede ser empleada como una fibra conductora o un catalizador para reacciones de oxidación. Se observó un recubrimiento policristalino sobre la fibra de vidrio, conformado por un arreglo continuo de agregados de CuO de 0.2-0.4 µm.
Palabras clave: CVD, deposición de CuO, fibra de vidrio, caracterización.
Introduction
Materials with a metallic coating have found application in a wide range of technological fields including solar cells [1], conducting polymers [2], superconductors [3], microelectronic devices [4] and recently they were also found to be effective as oxidation catalysts [5,6].
The utility of CVD to grow thin films is widely recognized in the semiconductor industry. The CVD process provides thin films with a high uniformity in chemical composition and it offers the ability to cover any geometric shape with a deposit of crystalline nanoparticles [7]. One advantage of CVD over other methods, is the ability to selectively deposit films onto a specific substrate area [8].
Cu(acac)2, has been used as a precursor for thin-film deposition of superconducting materials and metallic oxides by CVD techniques, due to its reasonable volatility among other advantages [9]. In the deposition zone, oxygen promotes the ligand removal from Cu(acac)2 and consequently, the deposition of the Cu site as indicated by Condorelli et al. [10]. Moreover, the deposition of Cu before oxidizing plays an important role as catalytic species facilitating the decomposition of the organic group at relatively low temperatures.
Copper oxides produced by CVD process; appear to have a great relevance as catalysts for oxidizing organic compounds at lower costs if compared with noble metals [5]. It has been found for instance, that Cu metal, Cu2O or CuO are not active for dehydration of 2-propanol. However the pre-reduced Cu2O or pre-oxidized Cu systems turn out to be very active [11]. A synergetic effect between oxidized and metallic Cu may explain such high activity. Specifically, CuO is a p-type semiconductor oxide considered to be used as an electronic component in the fabrication of solar cells [12]. Moreover, due to the similitude of the constructive planes of this structure and those of superconductive cuprates, this oxide is used as a model structure for the generation of high Tc superconducting oxides.
To our knowledge, there are no reports of metal phases deposited on fibreglass. Nevertheless, fibreglass is an interesting material to be used as support as that it offers the following advantages: low cost, flexibility and handling facility. Therefore, this communication describes for the first time, the chemical vapour deposition of copper oxides on a fibreglass substrate in order to generate a novel catalytic material or a structure to be integrated in an electronic device. In summary, the influence of sublimation temperature on the nature of deposited films was examined in the deposition reaction performed at 300 °C. This relatively low temperature was selected, as it is high enough to form the CuO phase from the copper precursor used.
Results and Discussion
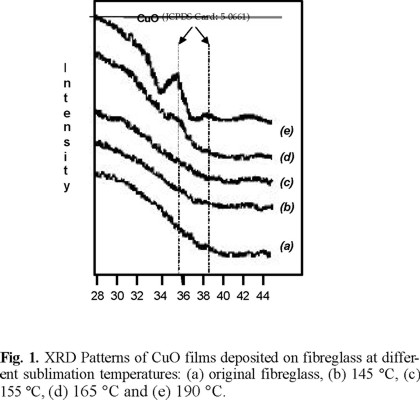
The X-ray diffraction patterns of the CuO film deposited on fibreglass are compared as the sublimation temperature was increased from 145 to 190 °C (Fig. 1). With an increase in the sublimation temperature above 165 °C two broad peaks are observed which can be attributed to the tenorite structure for CuO, according to (JCPDS card 5-0661) standard reported from the International Centre for Diffraction Data for the Powder sample. The relative intensity of the XRD peaks, 111 and 111 reflections with intensity of 100% at 2ϑ = 35.5° and 27% at 2ϑ = 38.7° are not in accordance with the JCPDS card since the iii peak for our sample is four times less intense. It is indicate a slight preferential orientation of the crystals to the (111) plane.
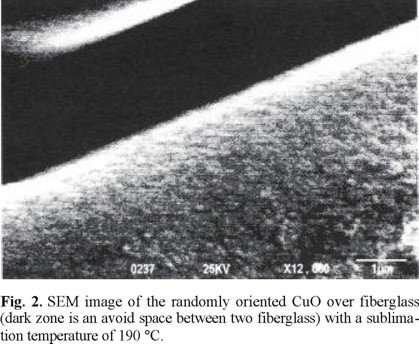
The SEM image of the surface morphology of the CuO film deposited on fibreglass at 300 °C and using a sublimation temperature of 190 °C is shown in (Fig. 2). The film deposited completely covers the substrate. The CuO film appears to be thin and continuous and its surface consists of separate small grains with diameters between 0.2 and 0.4 µm. The average sizes of the CuO grains could be close to the thickness of the film, which is composed by several layers of grains.
Since the peaks in (Fig. 1) are not very clear, it is not possible to know if these orientations are preferential, because it should be regarded that both the 111 and 111 reflections of CuO overlaps with other CuO reflections, 002 and 200 respectively. But if the temperature is lower, the diffraction peaks fade out. In all cases, the presence of the Cu2O phase was not observed in the XRD patterns. However the presence of Cu2O can not be ruled out. According to JCPDS card 5-0667, Cu2O has two strongest peaks at 2ϑ values of 36.42° (111) and 42.30° (200) respectively. Since the peaks in (Fig. 1-e) are very broad, it is quite possible that at least the peak around 36° also contains the 111 reflection of Cu2O. On the other hand the same spectrum, additionally shows a broad peak at about 42° around the position of the 200 reflection of Cu2O. Table 1 compiles the XRD results for the (Fig. 1).
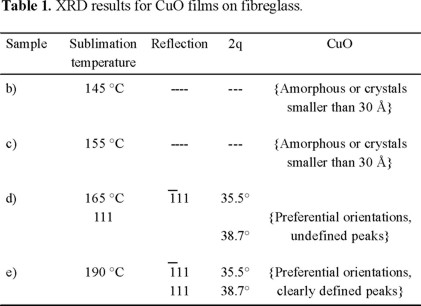
According to the X-ray diffraction patterns of the copper film deposited on fibreglass, only when the sublimation temperature is higher than 165 °C, the reflections characteristic of CuO were observed in accordance with the JCPDS card. Hence, in these samples (145-165 °C) either a homogeneous non-crystalline film of mixtures of Cu2O/CuO is formed or the crystalline particles are very small (less than 20 Å) to be observed by X-ray diffraction. Any of these propositions can explain the fading out of the peaks but only the first one could explain also the differences in colour among the samples.
Hence, we have prepared a fibreglass series with deposited CuO films as a new class of materials whose colours could be controlled mainly by the crystal size. This size was increased with the molar flow of Cu(acac)2 until a value of 69 Å (Scherrer formula) as shown by the broadening of the peaks of the sample (Fig. 1e). The films formed on the fibreglass ranged in colour from light brown for a low temperature of sublimation to dark brown for a high temperature of sublimation, this situation occurred when fibreglass was placed at the same position in the hot zone. Also the observed colours of the deposited films (brown) indicate the presence of Cu2O.
However, the changes in colour of the films can be due to the growth as well as to the shape of the nanostructures or quantum dots conforming the crystalline layer as established by Yoon et al. [13].
Experimental
The precursor, Cu(acac)2, purchased from Aldrich Chemical Co. was used without further purification. 8µm fibreglass from Corning Inc. (0.1g) was used as substrate after a cleaning treatment that consisted in washing the fibreglass using isopropyl alcohol and ultrasound bath. Gases were GC grade from Infra Co.
The film growth experiments were carried out using a horizontal-flow, atmospheric-pressure CVD hot wall reactor Fig. 3). The reactor consisted of a Pyrex glass tube (2 cm i.d. × 30-cm long) surrounded at each extreme by two furnaces, whose temperatures were monitored by means of Fe-constantan thermocouples.
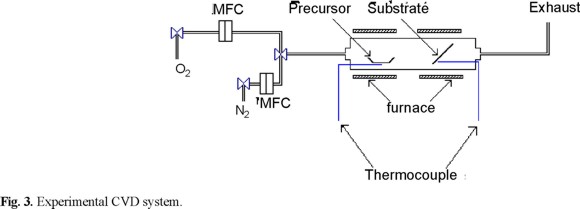
At the left side of the furnace, the precursor was sublimated at different temperatures and carried to the right side by an O2 flow of 30 ml min−1. At the right side of the furnace, the deposition of CuO took place at a temperature of 300 °C.
The molar flow rate of the precursor was determined by measuring the total quantity of Cu(acac)2 sublimated during the deposition time. It appears that this molar flow (fm) and the O2 / Cu(acac)2 molar ratio (y) do not have a lineal dependence with the sublimation temperature (Ts), e.g., Ts = 145 °C (fm = 2.9 × 10−8 mol min−1 , y = 4.2 × 107 ); Ts =155 °C (fm = 1.1 × 10−7 mol min−1 , y = 1.1 × 104); Ts = 165 °C (fm = 1.5 × 10−7 mol min−1 , y = 8.3 × 103); Ts = 190°C (fm = 2.2 × 10−7 mol min−1 , y = 5.6 × 103).
The CuO films on fibreglass were examined using several techniques. X-ray diffraction patterns (XRD) were obtained with a Siemens D500 diffractometer using Cu Kα radiation. A scanning electron microscope (SEM), model JEOL 1100, was used to observe the CuO films surface morphology.
Conclusions
The deposition of CuO on a fibreglass at a sublimation temperature higher than 165 °C and a high precursor flow promotes the formation of a single-phase with a (111) oriented polycrystalline structure. It was interesting to observe by the SEM technique, a continuous film when the oxide metal was deposited directly at a relatively low temperature. The surface morphology of deposited CuO films on fiberglass shows grains of micrometric sizes. Each of these grains might correspond to an aggregate of CuO crystallites. Fibreglass containing copper oxides could show remarkable oxidation properties compared to catalytic materials prepared by other techniques. Moreover, a fibreglass of amorphous silicon with a thin film of metal or metallic oxide is a potential material to be applied as an electronic device.
Acknowledgements
The financial support received by one of the authors (JRO) from the Programa Supera Anuies scholarship (0482) is acknowledged. The authors thank to Mr. Mariano Parga by the technical work.
References
1. Artaud, M.C.; Ouchen, F.; Martin, L.; Duchemin, S. Thin Solid Films 1998, 324, 115-123. [ Links ]
2. Nishio, S.; Okada, S.; Minamimoto, Y.; Okumara, M.; Matsuzaki, A.; Sato, H. Mol. Cryst. Liq. Cryst. 1997, 294, 35-40. [ Links ]
3. Popovici, D.; Cseremuzkin, G.; Meunier, M.; Sacher, E. Appl. Surf. Science 1998, 126, 198-204. [ Links ]
4. Kim, D.; Kim, T.; Chung, I.; Chung, C.W.; Lee, J. S. Integ. Ferroelec. 1997, 17, 67-76. [ Links ]
5. Chu, Hiu Ping.; Lei, Lecheng.; Hu, Xijun; Yue, Po-Lock. Energy & Fuels 1998, 12, 1108-1113. [ Links ]
6. Pranevicius, L.; Pranevicius, L. L.; Valatkevicius, P.; Valincius V. Surf. & Coat. Tecnol. 2000, 123, 122-127. [ Links ]
7. Okumara, M.; Nakamura S.; Tsubota, S.; Nakamura, T.; Azuma, M.; Haruta, M. Catal. Letters 1998, 51, 53-55. [ Links ]
8. Hampden-Smith, M. J.; Kodas, T. T. Chem. Vap. Deposition 1995, 1, 39-54. [ Links ]
9. Condorelli, G. G.; Malandrino, G.; Fragala, I. Chem. Mater. 1994, 6, 1861-1868. [ Links ]
10. Condorelli, G. G.; Malandrino G.; Fragala I., Chem. Mater. 1995, 7, 2096-2103. [ Links ]
11. Kung, H.H. Stud. Surf. Sci. and Catal. 1989, 45, 151-155. [ Links ]
12. Akomolafe, T.; Musa, A.O.; Carter, M.J. Solar Energy Mat. & Solar Cells 1998, 51, 305-309. [ Links ]
13. Yoon, S.; Moon, Y.; Lee, T.; Hwang, H.; Yoon, E.; Kim, Y.D. Thin Solid Films. 1999, 357, 81-91. [ Links ]














