Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Sociedad Química de México
versión impresa ISSN 0583-7693
Rev. Soc. Quím. Méx vol.44 no.2 Ciudad de México abr./jun. 2000
Investigación
Interaction of Copper(II) Dithiocarbamates with Hydrogen Peroxide
Roberto Cao,* Lázaro G. Pérez, Reynaldo Moya, Alicia Díaz
Laboratorio de Bioinorgánica, Facultad de Química, Universidad de La Habana, Zapata y G. Vedado, La Habana 10400, Cuba. Tel. 537-792145; Fax: 537-333502. e-mail: cao@fq.oc.uh.cu
Recibido el 3 de abril del 2000.
Aceptado el 18 de junio del 2000.
Abstract
The possible reaction of copper(II) dithiocarbamates with SOD-like activity and the ligands themselves with hydrogen peroxide was studied. Three dithiocarbamates (DTC) were selected: morpholyl (MorDTC) and those of L-glutamic acid (GluDTC) and L-proline (ProDTC). DTC salts reacted with H2O2, in a non-catalytic way, to form the corresponding disulfide and water. It was assumed that a sulfidyl radical is formed as intermediate. When these DTC form highly stable complexes with Cu(II), no interaction takes place. In the case of Cu(GluDTC)24− a partial dissociation of the complex provokes the reaction of the liberated ligand with H2O2, but disulfide is not formed, since apparently the intermediate reacts via redox with Cu(II), conducting to the total decomposition of the complex.
Key Words: copper(II) dithiocarbamates, hydrogen peroxide, redox, complex, decomposition.
Resumen
Se estudió la posible reacción de ditiocarbamatos de cobre (II) con peróxido de hidrógeno. Se seleccionaron tres ditiocarbamatos (DTC): morfolil (MorDTC), y los derivados de ácido L-glutámico (GluDTC) y L-prolina (ProDTC). Las sales de DTC reaccionaron con peróxido de hidrógeno de manera no catalítica, para formar el correspondiente disulfuro y agua. Se presupone que se forma un radical sulfidilo como intermediario. Cuando los DTC forman complejos altamente estables con cobre, no se lleva a cabo interacción. En el caso de Cu(GluDTC)24−, la disolución parcial del complejo provoca la reacción del ligando liberado con peróxido de hidrógeno, pero no se forma disulfuro, ya que aparentemente el intermediario reacciona via redox con Cu(II), conduciendo a la descomposición total del complejo.
Palabras clave: cobre (II) ditiocarbamatos, peróxido de hidrógeno, redox, complejo, descomposición.
To the memory of Jacobo Gómez Lara, a great friend, chemist and teacher
Introduction
Copper(II) bis(dithiocarbamates) are known to be very stable coordination compounds, with a quasi-planar structure [1]. These compounds were found to present SOD-like activity [2, 3]. They act as antioxidants in the dismutation of super-oxide radical, a reactive oxygen species (ROS), with the formation of dioxygen and hydrogen peroxide, another known ROS. It therefore resulted important to study the possible interaction that such compounds could present with this latest ROS.
Metal ions, mainly iron and copper, are known to react with hydrogen peroxide, H2O2, according to the so-called Fenton reaction:
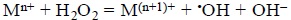
Or the Haber-Weiss reaction:
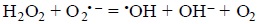
In both reactions hydroxyl radical, *OH, a very dangerous ROS, is formed.
In the case of copper, Cu(I) is assumed to be involved in the Fenton reaction, while Cu(II) could participate in the Haber-Weiss reaction.
The chemistry of copper salts can greatly differ from that of its coordination compounds, depending on the nature of the ligand coordinated to it and its oxidation state. Cu(I) tends to form tetrahedral complexes while Cu(II) square planar compounds. The nature of the donor atom of the ligand plays an important role in defining the redox properties of the copper complex.
It has been assumed that the reaction of hydrogen peroxide takes place through its coordination to copper, with the formation of a five coordinated adduct and a subsequent electron transfer [4]. Such mechanism assumes that a coordination site in copper(II) must be available and that the reduction of Cu(II) should also take place. Therefore, both steric and electronic conditions must be satisfied for a copper(II) complex to reaction with hydrogen peroxide.
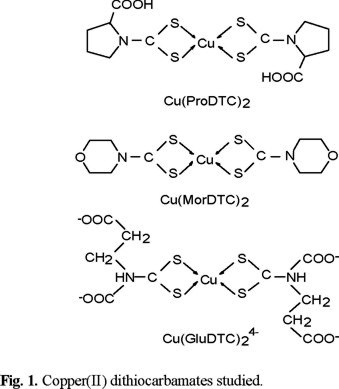
For this study three dithiocarbamates (DTC) were selected: morpholyl (MorDTC) and those of L-glutamic acid (GluDTC) and L-proline (ProDTC), two essential aminoacids. The goal of this paper is to define the possible reaction of copper(II) dithiocarbamates (Fig. 1) and the ligands themselves with hydrogen peroxide, H2O2. The reactions were followed spectrophotometrically.
Results and Discussion
Reaction of the dithiocarbamate salts. The first step was to define if the selected DTC, as their salts, were able to react with hydrogen peroxide. The reaction was followed spectrophotometrically. When mixed with hydrogen peroxide the three DTC react in the same way. Once the corresponding DTC and H2O2 were mixed in the quartz cell, the absorption bands at 240 nm, corresponding to the latter reagent, at 256-263 and 281-286 nm, of DTC, (Table 1) were observed to decrease continuously. At the same time a band at 317-327 nm appears and increases its absorbance during the first minutes and then decays (Table 1). This latest process take place together with the formation of a new band at 270-285 nm (Table 1). A Clarke type oxygen sensor was used in order to determine to the possible formation of dioxygen during this reaction. The result was negative indicating that H2O2 was reduced to H2O and not dismutated. Therefore, the DTC should have been oxidized during this process. Such type of reaction reminds the peroxidase process, an enzyme that requires a co-substrate to catalytic reduce H2O2:
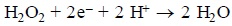
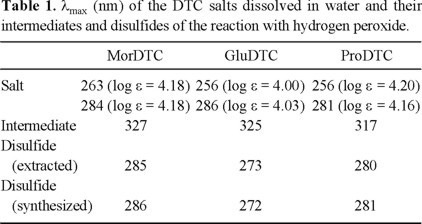
Since in our reaction no co-substrate is participating, the process is not catalytic and that is why it stops once one of the reagents is consumed, no matter the amount of the other added.
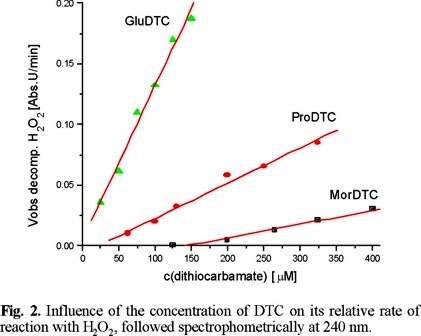
The rate of the reaction of DTC with H2O2 depends on both reagents. The observed relative rates (Fig. 2) indicate that MorDTC reacts more slowly while ProDTC is the fastest DTC. The fact that both DTC derived from L-aminoacids gave the highest rates of reaction could be interpreted as due to electrostatic attraction of their carboxylate group over H2O2 and its stabilization via H-bond.
After defining that the reaction between the DTC and H2O2 is a "peroxidase" type, it was important to determine the product of the oxidation of the DTC. The hypothesis was a disulfide, a known product of the oxidation of sulfur-containing compounds [5].
In order to confirm the formation of disulfide, the product of the reaction was studied. Once the reaction between DTC and H2O2 ended the resulting solution was mixed with chloroform. The organic phase was extracted and rotoevaporated. A white product was obtained for each DTC. The three disulfides were also obtained by the reaction of the corresponding DTC with iodine [6]:
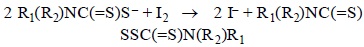
Only for the disulfide of MorDTC a high purity product was achieved. The NMR-1 3C spectra of the disulfides obtained by extraction and synthesis with iodine only differed in less than 0.18 ppm in their chemical shifts. The high coincidence between the compounds obtained by both methods (Table 1) indicates that the product of the interaction of DTC with H2O2 is the corresponding disulfide:
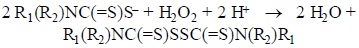
The band at 317-327 nm, that is initially formed to then disappear, should correspond to the formation of an intermediate, presumably a radical formed by electron transfer from H2O2 or hydroxyl radical formed by its homolytic disruption:
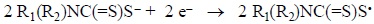
Two of such sulfidyl radicals, R1(R2)NC(=S)S*, could finally bond together by pairing their single electrons to give the corresponding disulfide. Unfortunately, we have no evidence to confirm this mechanism and previous studies on the formations of disulfides give no light in this direction.
Reaction of the copper(II) bis(dithiocarbamates). The stability constant of CuMorDTC)2, log β2 = 26.03 [1] is extremely high, while less stable for Cu(GluDTC)24−, log β2 = 19.35 [7]. The stability of Cu(ProDTC)2 has not been determined but all experimental evidences indicate that it is more stable than Cu(GluDTC)24−. Cu(MorDTC)2 and Cu(GluDTC)24− are lowly soluble in water, but Cu(ProDTC)2 is widely soluble.
In order to have a comparison of the structures of the three DTC complexes their EPR spectra were recorded. Due to the very low solubility in water of Cu(MorDTC)2 and Cu(GluDTC)24−, their EPR spectra could only be recorded in solid state. The EPR spectra of the complexes (Table 2) indicate that Cu(MorDTC)2 and Cu(ProDTC)2 present very similar structures, characterized by a high planarity. In the three cases the ground state corresponds to the dx2-y2 orbital since g// > g⊥ > ge (Table 2). The solid Cu(GluDTC)24− is EPR silent at both room temperature and temperatures as low as 77 K. When recording with a very high gain, a weak signal is observed with g1 = 2.133, g2 = 2.066, and g3 = 2.016. This signal is probably due to the monomeric form of the complex and indicates that it has a very distorted structure. The calculated G value for this latest complex is 3.2, indicating an anti-ferromagnetic interaction, probably through carboxylate bridging. This polymeric form could explain the extremely low solubility of this anionic complex.
The cyclic voltammograms of the three complexes gave Cu(II)/Cu(I) Ep values of −0.42 V for Cu(MorDTC)2, −0.46 V for Cu(GluDTC)24−, and −0.54 V for Cu(ProDTC)2. Therefore the reduction to Cu(I) is thermodynamically not favored. Dithiocarbamates, although being sulfur-containing ligands do not stabilize Cu(I) since their ϖ-acceptor character is negligible.
Of the three complexes, Cu(MorDTC)2, Cu(ProDTC)2 and Cu(GluDTC)24−, only the latest reacted with H2O2, corresponding to the complex with a different structure and less stability.
Cu(ProDTC)2, the only water-soluble complex, was studied by EPR spectroscopy using DMSO as an efficient hydroxyl scavenger. The signals of Cu(II) happened to fall within the range of the signals of the DMPO-*OH adduct (3350-3400 G) and no evidence was obtained from this experiment.
The copper(II) bis(dithiocarbamates), with their known high planarity, sterically should favor the coordination of H2O2 to the metal ion. Such bond should have a σ character, due to the high participation of pure "p" orbitals of oxygen in the peroxide molecule. An axial σ-bonding of H2O2, expected to be very weak if it could take place, should not affect the redox properties of Cu(II). This could explain why no reaction between H2O2 and Cu(MorDTC)2 and Cu(ProDTC)2 takes place. What happens then with Cu(GluDTC)24− ?
In the reaction of Cu(GluDTC)24− with H2O2 a picture somewhat similar to the reaction of the DTC salts was observed. This similarity suggests that the reaction takes place previous dissociation of the complex. From the same beginning of the reaction the MLCT band at 432 nm decreases its absorbance. At the same time a band at 325 nm, as a shoulder, appears but after the first 3 min starts to decrease. This shoulder reminds the band observed in the interaction of the DTC salts with H2O2 (Table 1), which was interpreted as due to a sulfidyl intermediate. This intermediate is now threefold less stable. No band corresponding to the formation of disulfide was observed. Since the disulfide was not formed it could be expected that the sulfidyl radical reacted with free Cu(II), formed by the partial dissociation of the complex. Cu(II) would be reduced to Cu(I) and the sulfidyl radical reoxidized to DTC:
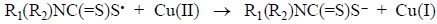
Such electronic interaction would complete the dissociation of the complex. At the end of the reaction only the absorbance of CS2 was observed indicating a total decomposition of both complex and ligand. Extraction with chloroform gave no disulfide phase.
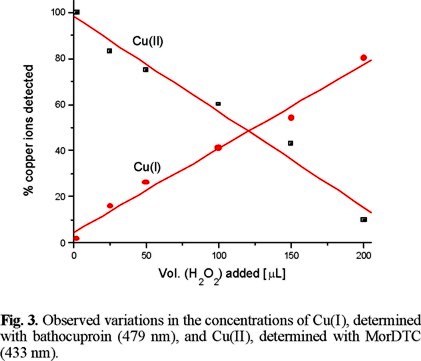
The assumed reduction of Cu(II) to Cu(I) was followed using bathocuproin, a specific reagent for Cu(I). The remaining presence of Cu(II) was monitored using MorDTC. A linear variation was observed for both copper ions, with the concentration of Cu(I) increasing in the same proportion that Cu(II) diminished (Fig. 3) as the amount of H2O2 added was increased. This result confirms the reduction of Cu(II) in Cu(GluDTC)24− as hydrogen peroxide is added.
Conclusions
DTC salts can react with H2O2, in a non-catalytic way, to form the corresponding disulfide. Apparently, a sulfidyl radical is formed as intermediate. When these DTC form highly stable complexes with Cu(II), such as in Cu(MorDTC)2 and Cu(Pro DTC)2, no interaction takes place. In spite of the fact that these square planar complexes are sterically available for the interaction with H2O2 in the apical position, the σ-electronic interaction is too weak to take place. In the case of Cu(GluDTC)24− a partial dissociation of the complex provokes the reaction of the liberated ligand with H2O2 but disulfide is not formed since apparently the intermediate reacts with Cu(II) in a redox fashion, conducting to a total decomposition of the complex.
Experimental
All reagents were purchased from Merck (except DMPO, from Sigma) and were of analytical quality. The three dithio-carbamate salts are trihydrated; the presence of three water molecule was established by thermogravimetric analyses.
Elemental analysis was performed on an Eager 200 analyzer. Copper was determined spectrophotometrically at 479 nm using bathocuproin. The electronic spectra were acquired with an Ultrospec III (Pharmacia-LKB) spectrophotometer. The IR spectra were recorded on a PU9600 FT-IR spectrometer (Philips). The 1H- and 13C-NMR spectra were performed on a Bruker AC-250F spectrometer. Dioxane (13C) and DSS (1H) were used as internal references. The EPR spectra were recorded on a Bruker EMX 812 spectrometer. The formation of hydroxyl radicals was intended to be studied by EPR using 5,5-dimethyl-1-pyrroline N-oxide, DMPO, as its scavenger for 0.4 mM Cu(ProDTC)2 and 2 mM DMPO according the method reported in [8].
Cyclic voltammetric experiments were carried out on a Yanaco P8 polarographic analyzer using a three-electrode cell. Working electrode: static mercury drop electrode; Ag/sta AgCl in DMSO, not calibrated. The complex concentration was held at 0.1 M in DMSO and the supporting electrolyte was 0.1 M LiNO3. The scan was kept constant at 0.1 V/s. Prior to each experiment the solutions were deoxygenated using pure nitrogen gas.
Synthesis of the sodium salt of morpholyl dithiocarbamate trihydrate (MorDTC) [2].
An ethanol solution of morpholine was added dropwise to an ethanol solution of CS2 at 0-5 ºC (morpholine:CS2 molar ratio of 1:1). The resulting mixture was treated with diethyl ether and an aqueous solution of NaOH for a CS2:NaOH molar ratio of 1:1. The product was filtered, washed and recrystalized from ethanol. Yield 52 %. m.p.>300 ºC. UV (H2O) λ (log ε) 263 (4.18) nm (CSS ϖ-ϖ*); 284 (4.18) nm (NCS ϖ-ϖ*); IR (KBr) νmax: 1460 (νC=N), 981 (νC-S), 542 cm−1 (νC-S + δSCS); 1H NMR (250 MHz, D2O): δ 4.38 (t, 4H, -OCH2-, JH-H = 5.1 Hz); 3.77 (t, 4H, -NCH2-, JH-H = 4.9 Hz). Anal. C, 25.20; H, 3.63; N, 5.69. Calcd. for C5H8NNaOS2. 3H2O: C, 25.10; H, 3.37; N, 5.85.
Synthesis of the barium salt of L-proline dithiocarbamate trihydrate (ProDTC). 10 mmol (1.1 g) of L-proline in aqueous solutions (10 mL) was mixed with 12 mmol (2.5 g) of BaCl2.2H2O, then 20 mL of NH3 (25%) was added. This solution was heated and stirred, and finally filtered. The aqueous solution was cooled and vigorous magnetically stirred while 1 mL of CS2 was added dropwise. Then, 30 mL of ethanol were added and stirred for 2.5 h, after which other 30 mL of ethanol were added. The solution was kept in a refrigerator for 24 h. The formed white precipitate was filtered and washed with ethanol and ether. The compound was purified by dissolving it in water and precipitated in ethanol. Yield 60%. m.p.>300 ºC. UV (H20) λ (log ε) 256 (4.20) nm (CSS ϖ-ϖ*) 281 (4.16) nm (NCS ϖ-ϖ*); IR (KBr) νmax 1557 (νas COO -), 1397 (νs COO -), 1187 (ν C-N), 1009 (νas C=S), 664 cm−1 (νs C=S); 1H NMR (250 MHz, D2O) δ 2.02 (m, 2H, H3-CH2), 2.30 (m, 2H, H2-CH2), 3.88 (m, 2H, H4-CH2); 4.80 (m, 1H, H1-CH). 13C NMR (63 MHz, D2O) δ 27.20 (C3-CH2), 33.93 (C2-CH2), 58.11 (C4-CH2), 71.18 (C1-CH), 182.76 (C5-COO), 207.74 (C6-CSS). Anal. C, 18.60; H, 3.73; N, 3.49;. Calcd. for C6H7BaNO2S2.3H2O: C, 18.94; H, 3.44; N, 3.68.
Synthesis of the barium salt of L-glutamic acid dithiocarbamate trihydrate (GluDTC). The procedure used is similar to that reported above for ProDTC. Yield 62 %. m.p.>300 ºC. UV (H20) λ (log ε) 256 nm (4.00) nm (CSS ϖ-ϖ*); 286 (4.03) nm (NCS ϖ-ϖ*); IR (KBr) νmax 1540 (νas COO -), 1403 (νs COO -), 1110 (ν C-N), 957 (νas C=S), 630 cm−1 (νs C=S); 1H NMR (250 MHz, D2O): δ 3.25 (t, CH); 13C NMR (63 MHz, D2O) δ 71.36 (CH), 183.72, 187.26 (COO), 215.93 (CSS). Anal. C, 16.10; N, 3.19; O, 14.03. Calcd. for C12H12Ba3N2O8 S4.3H2O: C, 15.90; N, 3.09; O, 14.12.
Synthesis of bis(morpholyldithiocarbamate)copper(II) trihydrate (Cu(MorDTC)2). The copper(II) complex was prepared by slow addition of an aqueous solution of CuCl2.2H2O over an aqueous solution of MorDTC in a 1:2 molar ratio. A dark brown precipitate was immediately formed, filtered off and washed with water, ethanol and diethyl ether. Yield 99 %. UV (DMSO/H2O): UV-Vis (H2O) λmax (log ε, transition) 433 (4.10, M→L) nm, 640 (shoulder, d-d) nm; IR (KBr) νmax 1485 (νCDTC-N), 1191 (νC-N), 1009 cm−1 (νasC-S). Anal. C, 27.36; H, 3.90; N, 6.25; Cu, 14.26. Calcd. for C10H16CuN2O2 S4.3H2O: C, 27.17; H, 3.65; N, 6.34; Cu, 14.37.
Synthesis of bis(L-prolinedithiocarbamate)copper(II) (Cu(ProDTC)2). The copper(II) complex was prepared by slow addition of an aqueous solution of ProDTC over an aqueous solution of CuCl2.2H2O in a 2:1molar ratio. Then, ether was added, followed by a dropwise additional of a 0.1 M HCl solution. The ether phase, containing the extracted complex, was separated and dried in a rotatory vacuum evaporator. Yield ≈ 50%. UV-Vis (H2O) (log ε, transition) 269 (4.28, L) nm, 432 (4.12, M→L) nm, 640 (shoulder, d-d) nm; IR (KBr) νmax 1477 (νCDTC-N), 1156, 1185 (νC-N), 1716 (νCOOH), 956 (νasC-S), 678 cm−1 (νsC-S). Anal. C, 32.36; H, 3.97; N, 6.05; Cu, 14.20. Calcd. for C12H16CuN2O4S4: C, 32.46; H, 3.60; N, 6.30; Cu, 14.31.
Synthesis of the barium salt of bis(L-glutamic acid dithiocarbamate)copper(II) trihydrate (Cu(GluDTC)24−). The copper(II) complex was prepared by a procedure similar to that of Cu(MorDTC)2. Yield ≈ 85 %. UV-Vis (DMSO/H2O) (log ε, transition) 432 (3.53, M→L) nm, 638 (shoulder, d-d) nm. IR (KBr) νmax 1469 (νCDTC-N), 1135 (νC-N), 1586, 1498, 1322 (νCOO-), 988 cm−1 (νasC-S). Anal. C, 17.13; H, 1.88; N, 3.50; Cu, 7.44. Calcd for C12H12Ba2CuN2O8S4: C, 17.31; H, 1.45; N, 3.36, Cu, 7.63.
References
1. Sastri, V. S.; Aspila, K. J. Can. J. Chem. 1969, 47, 2320-2325. [ Links ]
2. Cao, R.; Fragoso, A.; Villalonga, R. Monatsh. Chem. 1996, 127, 775-782. [ Links ]
3. Cao, R.; Travieso, N.; Fragoso, A.; Villalonga, R.; Díaz, A.; Martínez, M. E.; Alpizar, J.; West, D. X. J. Inorg. Biochem. 1997, 66, 213-217. [ Links ]
4. Bonomo, R. P.; Marchelli, R.; Tabbí, G. J. Inorg. Biochem. 1995, 60, 205-218. [ Links ]
5. Moldéus, P.; Cotgreave, I. A.; Berggren, M. Respiration 1986, 50, 31-42. [ Links ]
6. Birko, B. M. Ditiokarbamati. Nauka. Moscow. 1984. [ Links ]
7. Cao, R.; Sanchiz, J.; Mederos, A. VII Spanish-Italian and Mediterranean Congress on Thermodynamics of Metal Complexes. Granada. 1996. [ Links ]
8. Saryan, L. A.; Mailer, K.; Krishnamurti, C.; Antholine, W. Biochem. Pharmacol. 1981, 30, 1595-1604. [ Links ]














