Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de la Sociedad Química de México
Print version ISSN 0583-7693
Rev. Soc. Quím. Méx vol.44 n.2 Ciudad de México Apr./Jun. 2000
Investigación
Infrared Spectroscopy and X-Ray Diffractometry Assessment of Order-Disorder in Oxide Minerals (Mn/Fe)(Nb/Ta)2O6
M. S. Augsburger,1 J. C. Pedregosa,1* and G. M. Sosa2
1 Laboratorio de Química Inorgánica (PID-CONICET), Facultad de Química, Bioquímica y Farmacia, Universidad Nacional de San Luis, Chacabuco y Pedernera, D5700, San Luis, Argentina. e-mail: jpedreg@unsl.edu.ar.
2 Departamento de Geología, Facultad de Ciencias Físico, Matemáticas y Naturales, Universidad Nacional de San Luis, Chacabuco y Pedernera, D5700, San Luis, Argentina.
Recibido el 6 de marzo del 2000.
Aceptado el 9 de junio del 2000.
Abstract
The degree of cation order of four columbite-tantalite from San Luis Range (Argentina) was evaluated from X-ray diffractometry and FTIR spectroscopy. Data were compared with those obtained by Mössbauer spectrometry. Based on structural evidences, a general assignment for the Nb/Ta-O6 and Fe/Mn-O6 octahedra internal vibrations is proposed.
Key Words: columbite-tantalite, FTIR spectroscopy, X-ray diffractometry, Mössbauer.
Resumen
Se evaluó el grado de orden catiónico de cuatro columbita-tantalitas de las Sierras de San Luis (Argentina) a partir de difractometría de Rayos X y espectroscopía FTIR. Los resultados fueron comparados con los obtenidos por espectroscopía Mössbauer. Se propone una asignación general para los modos internos de vibración de los octaedros Nb/Ta-O6 y Fe/Mn-O6 en base a evidencias estructurales.
Palabras clave: columbita-tantalitas, difractometría de Rayos X, espectroscopía FTIR, Mössbauer.
Dedicated in memoriam to Professor Jacobo Gómez-Lara
Introduction
Columbite-tantalite group minerals are the main source of niobium and tantalum oxides (Nb2O5 and Ta2O5) which find important applications in electronic and optical industries [1-3]. Columbite-tantalite has the general formula AB2O6 and shows a wide compositional range with the A position mostly occupied by Fe(II), Mn(II) and to a lesser extent by Mg(II), and the B position mainly occupied by Nb(V), Ta(V) and subordinately by Ti(IV), W(VI) and Sn(IV) [4]. The end members of these orthorhombic minerals are ferrocolumbite (FeNb2O6), manganocolumbite (MnNb2O6), manganotantalite (MnTa2O6) and magnocolumbite (MgNb2O6), with their crystal structure related to that of brookite (TiO2) or α-PbO2. The atoms at the A and B sites in the crystal structure of columbite are coordinated to six oxygens which form distorted octahedra. These octahedra are stacked along the crystallographic a-axis in a sequence of ABBABB layers, in which A-type octahedra share corners while B-type octahedra share edges. Although the 3d transition metals occupy mainly the A site, there exists a considerable degree of cation occupation disorder between sites A and B; i.e. Fe, Mn, Nb or Ta are found to occupy both sites A and B in different proportions. Different cationic occupancy of the sites from one end-member to another leads to changes of the unit cell dimensions and to distortions in the oxygen octahedra.
Disorder evaluation of these oxide minerals is important because it has considerable effects on the magnetic properties [5]. In general, the columbite-tantalite cation order-disorder has been estimated from X-ray powder diffraction data (XRPD) or, recently by Mössbauer spectroscopy (MS). Other criteria have been established to estimate the degree of cation order by FTIR.
Evaluating X-ray powder diffraction data (XRPD), Komkov [6] found that the a/c ratio of these minerals significantly varies with changing Mn/Fe ratio, where a and c are the lattice parameters. Cerný and Ercit [7] observed that in columbite-tantalite with variable composition the degree of order increases with higher Mn-content. Ercit [8] derived an empirical equation to estimate the degree of cation order in columbite: % order (± 5%) = 1727 - 941.6(c - 0.2329a).
Mössbauer spectroscopy of 57Fe has been successfully applied in mineral studies to find the oxidation state and iron occupation sites [9].
Other criteria have been established to estimate the degree of cation order by FTIR. A sharp band for ν3 vibrational mode (Nb/Ta-O) on FTIR spectra indicate a high degree of order in the lattice [10].
Four columbite-tantalite samples from different pegmatite bodies from the San Luis Range (Argentina) have been analyzed by X-ray powder diffractometry (XRPD) and infrared spectroscopy (FTIR). The X-ray powder diagrams were attained to characterize the structural state and the degree of cation order. The columbite-tantalite FTIR spectra are reported here for the first time. The infrared absorption bands could be assigned to the internal vibrations of the Nb/Ta-O and Fe/Mn-O bonds. Furthermore, it was possible to estimate a comparative degree of order. The results are consistent with those obtained from Mössbauer spectra.
Results and Discussion
The XRPD patterns were indexed to the Pbcn 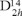 , Nº 60), space group, with Z = 4. A modified version of the program PIRUM [11] was used for determining the unit-cell parameters and the cation order was calculated following Ercit´s formula [8] (see Table 1). Our samples have a degree of order between 84 % (sample Mc) and 41% (sample M4). The high degree of order observed in sample M1 (manganocolumbite) is in good agreement with the high percentage of Mn present in the mineral, as proposed by Cerny et al. [6].
, Nº 60), space group, with Z = 4. A modified version of the program PIRUM [11] was used for determining the unit-cell parameters and the cation order was calculated following Ercit´s formula [8] (see Table 1). Our samples have a degree of order between 84 % (sample Mc) and 41% (sample M4). The high degree of order observed in sample M1 (manganocolumbite) is in good agreement with the high percentage of Mn present in the mineral, as proposed by Cerny et al. [6].
The FTIR spectra and band assignments are displayed in Fig. 1 and Table II, respectively. In spite of the broad absorption bands and low definition it was possible to sort out the bands corresponding to Ta/Nb-O and Mn/Fe-O modes.
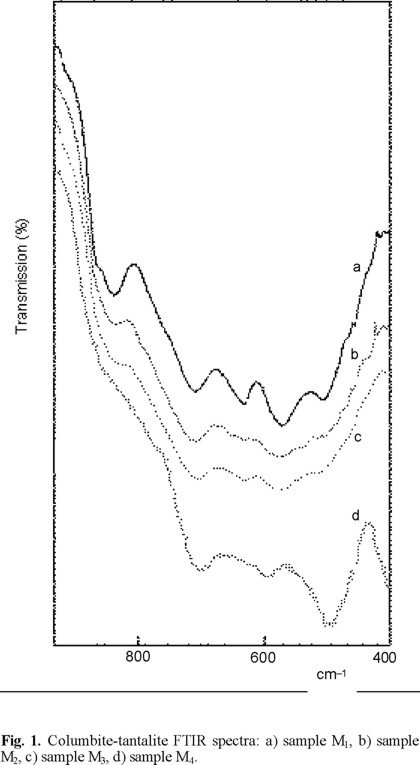
By comparison with the IR-absorption bands of synthetic similar species [10, 12-15] most important vibrational modes could be identified. Some of these synthetic species correspond to LiNbO3 and H-Nb2O5 crystals which form NbO6 octahedra. The essential difference between both compounds is that in LiNbO3 the NbO6 octahedra share only corners while in H-Nb2O5 the NbO6 octahedra share both edges and corners, as in columbite-tantalite with disorder between sites A and B [14,15]. Our assignment is in good agreement with H-Nb2O5.
Based on these structural evidences the bands at around 700 and 630 cm−1 have been assigned to the (3 mode in the corned-shared NbO6 octahedron and the additional two bands at around 830 and 500 cm−1 are attributed to the edge-shared NbO6 octahedron. The sharper band (sample M1, Fig. 1) indicates a higher degree of order of the crystal lattice and is consistent with the high structural order (84%) calculated from XRPD data.
The bands at 600-500 cm−1 correspond to the ν3 and ν4 vibrational modes of the Fe/MnO6 octahedra. The expected splitting by the symmetry lowering effects in the lattice in relation to the point group of the octahedral species, is not observed in our spectrum. Besides, there are no significant couplings between the octahedra vibrational modes in condensed layers A and B such as in other species with large cations [16].
In the low frequency region (500-400 cm−1), the vibrational behavior strongly depends on the nature of the divalent cation and is mainly influenced by Fe (II) and Mn (II) in this case. The absorption bands in this part of the spectrum are of complex origin and correspond to contributions of bending and lattice modes.
A better definition of the spectra was not attained at lower temperature. Spectra were recorded for sample M3 (Fig. 2) at room temperature and at 80 K, using a liquid nitrogen cooled cell (RIIC-Beckman). The expected spectral splitting at low temperature normally caused by the symmetry lowering effects in the crystal lattice, i.e. in relation to the point group of the octahedra, has not been observed.

Conclusions
X-ray and infrared spectroscopy are powerful methods to identify and characterize the structural state of columbite-tantalite in a simple and rapid way. The results of both methods are coherent and indicative for the degree of order of the crystal structure (cationic distribution and distortion). Our results also concurr with the degree of order obtained by the interpretation of Mössbauer spectra .
Experimental
The XRPD patterns were obtained in a Rigaku D-MAX III diffractometer using Ni-filtered CuKα radiation (λ = 1.5418 Å) and a scan speed of 2° 2ϑ/min. NaCl was used as an internal calibration standard. FTIR spectra in the 4000-400 cm−1 range were recorded with a Bruker IFS25 spectrophotometer, using the KBr pellet technique. The spectral resolution is better than 2 cm−1 between 4000 and 2000 cm−1 and better than 1 cm−1 in the spectral range under 2000 cm−1.
Acknowledgements
This work was supported by CONICET (PICT 4929/96 and PICT 0201) and Universidad Nacional de San Luis (Secretaría C y T, Proyecto 7707 and Proyecto 348903).
References
1. Albrecht, W.W., in: Special Publication Nº7 of the Society for Geology Applied to Mineral Deposits: Lanthanides, Tantalum and Niobium, Springer-Verlag, Berlin, 1989. [ Links ]
2. Clarke, D. R.; Daumling, M., in Materials Science and Technology, Structure and Properties of Ceramics, Vol. 11, Ed., VCH Publishers Inc., New York, 1994. [ Links ]
3. Smart, L.; Moore, E., Solid State Chemistry, An Introduction, Ed., Chapman and Hall, London, 1992. [ Links ]
4. Graham, J.; Thornber, M. Am. Mineral. 1976, 59, 1024. [ Links ]
5. Zawislak, L. I.; Antonietti, V.; da Cunha, J. M.; dos Santos, C. A. Solid State Commun. 1997, 101, 767. [ Links ]
6. Komkov, I. A. Dokl. Akad. Nauk. Earth Science Sect. 1970, 194, 434. [ Links ]
7. Cerný, P.; Ercit, T. S. Bull. Mineral. 1985, 10, 499. [ Links ]
8. Ercit, T. S. The simpsonite paragenesis: The crystal chemistry and geochemistry of extreme Ta fractionation. Ph. D. Diss., University of Manitoba, Canada, 1986. [ Links ]
9. Augsburger, M. S.; Pedregosa, J. C.; Sosa, G. M.; Mercader R. J. Solid State Chem. 1999, 143, 219. [ Links ]
10. Choisnet, J.; Nguyen N.; Raveau, B. J. Solid State Chem. 1978, 26, 83. [ Links ]
11. Werner, P. Ark. Kemi. 1969, 315,13. [ Links ]
12. Fakhfakh, M.; Verbaere, A.; Jouini, N. Eur. J. Solid State Inorg. Chem. 1992, 29, 563. [ Links ]
13. De Araujo, E. B.; de Paiva, J. A.; Freitas, J.A.; Sombra, A. S. J. Phys. Chem. Solids 1998, 59, 689. [ Links ]
14. Tatsumisago, M.; Hamada, A.; Minami, T.; Tanaka, M. J. Non-Cryst. Solids 1983, 56, 423. [ Links ]
15. Fukimi, K.; Sakka, J. J. Mater. Sci. 1988, 23, 2819. [ Links ]
16. Augsburger, M. S.; Pedregosa, J. C. J. Phys. Chem. Solids. 1995, 56, 1081. [ Links ]














