Servicios Personalizados
Revista
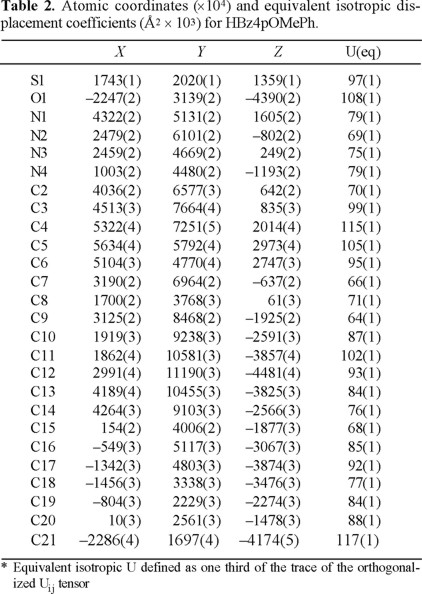
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Sociedad Química de México
versión impresa ISSN 0583-7693
Rev. Soc. Quím. Méx vol.44 no.2 Ciudad de México abr./jun. 2000
Investigación
Crystal and Molecular Structure of 2-Benzoylpyridine N4-p-methoxyphenylthiosemicarbazone
Jesús Valdés-Martínez,1* Rubén A. Toscano,1 Douglas X. West,2* Jack J. Ingram III2 and Gordon A. Bain2
1 Instituto de Química, Universidad Nacional Autónoma de México, Circuito Exterior, Ciudad Universitaria, Coyoacán 04510, México, D.F. Phone: 56224514. E-mail: jvaldes@servidor.unam.mx.
2 Department of Chemistry, Illinois State University, Normal, IL 61790-4160, USA.
Recibido el 28 de abril del 2000.
Aceptado el 19 de mayo del 2000.
Abstract
2-benzoylpyridine N4-p-methoxyphenylthiosemicarbazone, HBz4pMeOPh, crystallizes with a Z arrangement of N1 and N2 with respect to C7-N2, which allows for the N3-H3A...N1 hydrogen bond and a six-membered ring. In addition, a 5-membered ring involving a second hydrogen bond, N4-H4A...N2 results in the N2 and S1 E conformation with respect to N3-C8. Intramolecular C-H...O and C-H...S hydrogen bonds create supramolecular chains.
Key Words: thiosemicarbazones, crystal structure.
Resumen
La 2-benzoilpiridina N4-p-metoxifeniltiosemicarbazona, HBz4pMeOPh, cristaliza con un arreglo Z de N1 y N2 con respecto a C7-N2 lo que permite la formación del enlace de hidrógeno N3-H3A...N1 en un anillo de seis miembros. Adicionalmente se forma un anillo de 5 miembros el cual involucra un segundo enlace de hidrógeno, N4-H4A...N2 del cual resulta una conformación E de N2 y S1 con respecto a N3-C8. Se producen cadenas supramoleculares debido a los enlaces de hidrógeno C-H...O y C-H...S.
Palabras clave: tiosemicarbazonas, estructura cristalina.
A la memoria de Jacobo, maestro y amigo
Introduction
Heterocyclic thiosemicarbazones have been shown to possess a broad spectrum of pharmacological activities [1]. It has been proposed, based on NMR studies, that these molecules exist in solution in at least three different isomeric conformations: E, E' and Z [2] (Fig. 1).
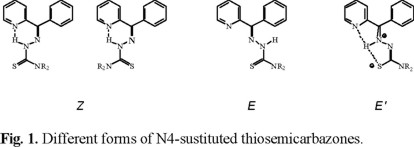
The crystal structure of the E isomer with respect to C7-N2 was reported for 5-hydroxy-2-formylpyridine thiosemicarbazone, H5OHFo4DH [3], 2-formylpyridine N4-methylthiosemicarbazone, HFo4M [4], and 2-acetylpyridine N4-ethylthiosemicarbazone, HAc4E [4]. The Z conformation was observed in 2-formylpyridine 3-azabicyclo[3.2.2]nonylthiosemicarbazone, HFobcn, the E' isomer has been reported for 2-acetylpyridine 3-hexamethyleneiminylthiosemicarbazone, HAchexim [4], and earlier for 2-acetylpyridine 3-azabicyclo[3.2.2] nonylthiosemicarbazone, HAcbcn [5]. Although there have been reports of 2-formyl- and 2-acetylpyridine thiosemicarbazones, particularly those without substituents at N4, as well as two copper(II) complexes of 2-benzoylpyridine N4-substituted thiosemicarbazones [6], there have only three structures of uncomplexed 2-benzoylpyridine thiosemicar-bazones reported. 2-benzoylpyridine N4-methyl-N4-phenylthiosemicarbazone, HBz4MePh, was found to be in the Z conformation with respect to C7-N2 [7], as were 2-benzoylpyridine 3-piperidyl and 3-hexamethyleminimylthiosemicarbazone [8], HBzpip and HBzhexim respectively, but there was a difference in their conformation with respect to the N-C(S) bond. Because of this 4th structural modification of thiosemicarbazones, we decided to look for an additional example and report here the crystal structure of 2-benzoylpyridine N4-p-methoxyphenylthiosemicarbazone, HBz4pMeOPh.
Results and Discussion
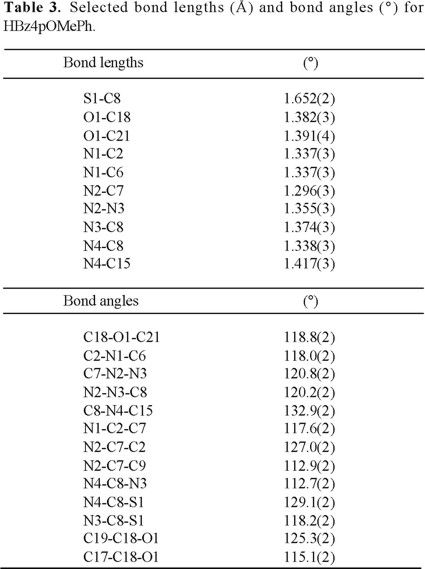
The shape of the molecule and the atomic numbering are indicated in Fig. 2. Final atomic parameters are given in Table 2 and selected bond distances and angles in Table 3. N1 and N2 present a Z conformation with respect to the C7-N2 bond, and N2 and S1 an E conformation with respect to N3-C8. The conformation of the ligand may be explained by the intramolecular hydrogen bonds: N3-H3A...N1, and N4-H4A...N2. The thiosemicarbazone backbone, N2-N3-C8-S-N4 (TSCB), is nearly planar, with a mean plane deviation of 0.008 Å. The pyridine ring forms an angle of 13.24(6)° with the TSCB. Both phenyl groups, on C7 and N4, deviate from the TSCB plane, with angles of 45.3(1)° and 14.1(1)°, respectively. The deviation of the former ring with respect to the plane of the TSCB may be understood by considering the C3H3-C14H14 repulsions of the pyridyl and benzoyl rings.

Although according to Etter's hydrogen-bond rules for organic compounds [9], six-membered-ring hydrogen bonds are preferred to intermolecular hydrogen bonds, it is interesting to mention that the six-membered-ring hydrogen bond N3-H3A...N1 is not present in HFo4M, HAc4E [4] or H5OHFo4DH [3]. Several facts indicate a greater delocalization when this 6-membered ring is present in the molecule: the N2-N3 bond distances in the Z isomers are shorter, 1.355(4) and 1.321(8)Å for HBz4pMeOPh and HFobcn, respectively, than the ones observed in the E isomer ligands, HFo4M and HAc4E 1.375(2) and 1.370(2)Å, respectively [4]. The present compound has C7-N2-N3 and N2-N3-C8 bond angles of 120.6(4) and 120.4(5)° respectively, and the C7-N2-N3-C8 dihedral angle is 180(1)°. Another consequence of this ring is the opening of the C2-C7-N2 angle, with values of 127.3(5)° in HBz4pMeOPh and 132.2(7)° in HFobcn, compared to 120.5(2)° and 114.7(2)° in HFo4M and HAc4E respectively [4].
The E conformation between S1 and N2 is explained by considering the hydrogen bond between N4-H...N2. The five-membered ring hydrogen bond formed is also rather planar, with a N2-N3-C8-N4 dihedral angle of 2(1)°. In the present compound the S1-C8-N4 angle has a value of 129.2(5)°. This distortion may be a consequence of the O1...H5-C5 intermolecular hydrogen bond, vide infra.
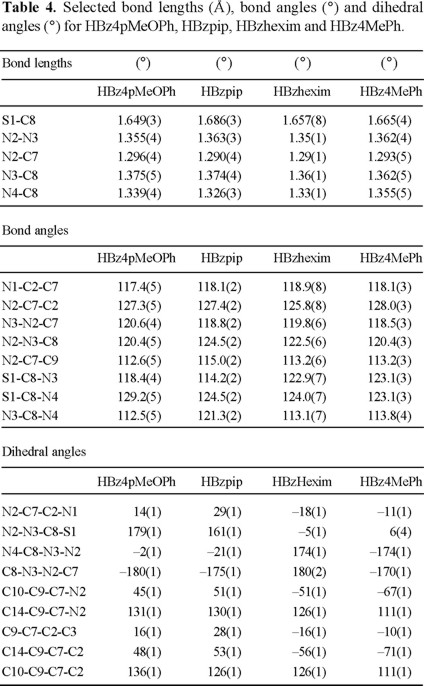
When the structure of the four HBzTSC molecules reported are compared, see Table 4, maybe the most significant differences are related to the greater planarity in the TSCB in HBz4pMeOPh. The C8-S1 bond length is shorter, the in N3-N2-C7 and N2-N3-C8 are near 120° and the values of N2-N3-C8-S1, N4-C8-N3-N2 and C8-N3-N2-C7 are closed to 180 or 0°. These differences may be explained by the two intramolecular hydrogen bonds present only in HBz4pMeOPh.
As long as the hydrogen atoms on the nitrogens are involved in intramolecular hydrogen bonds, and there are no other acidic hydrogen atoms, this molecule is a good candi-date for the study of C-H ... O and C - H ... S hydrogen bonds. There is a C5 - H5 ... O1 contact within the accepted distances (3.0-4.0 Å) and angles (90 - 180°) for a C - H ... O hydrogen bond [10]. The distance between C5 and O1 of an adjacent molecule (1 + x, y, 1 + z) is 3.390(4) Å. The C5 - H5 and H5 ... O1 distances are 0.91(3) and 2.61(2)Å, respectively, with a C5-H5...O1 angle of 144(2)°. Two of the three hydrogen atoms of C21 hydrogen bond to the same S1 atom at (-x, -y, -z). The C21 ... S1 distance is 3.518(3) Å, the H...S1 distances for H21B and H21C are 3.12(3) and 3.06(3) Å, respectively, and the C21 - H distances are 0.94(3) and 0.96(3) Å for H21B and H21C, respectively. The C21 - H ... S1 bond angles for H21B and H21C are 108(2) and 111(2)°, respectively. The combination of these hydrogen bonds produce macromolecular chains as shown in Fig. 3.

Experimental
HBz4pMeOPh was prepared as described previously [6, 11], crystals were grown by slow diffusion (−10 °C) of diethyl ether into chloroform solution. The structure determination summary is shown in Table 1. All hydrogen atoms were located in the Fourier map difference and refined isotropically.

References
1. Liberta, A. E.; West, D. X. BioMetals 1992, 5, 121-126. [ Links ]
2. West, D. X.; Mokijewski, B. L.; Gebremedhin, H.; Romack, T. J. Transition Met. Chem. 1992, 17, 384-386. [ Links ]
3. Palenik, G. J.; Rendle, D. F.; Carter, W. S. Acta Cryst. 1974, B30, 2390-2395. [ Links ]
4. West, D. X.; Bain, G. A.; Butcher, R. J.; Jasinski, J. P.; Li, Y., Pozdniakiv, R.Y.; Valdés-Martínez, J.; Toscano, R. A.; Hernández- Ortega, S. Polyhedron 1996, 15, 665-674. [ Links ]
5. West, D. X.; Ahrweiler, P. M.; Ertem, G.; Scovill, J. P.; Klayman, D. L.; Flippen-Anderson, J. L.; Gilardi, R., George, C.; Pannell, L. K. Transition Met. Chem. 1985, 10, 264-270. [ Links ]
6. West, D. X.; Ives, J. S.; Krejci, J.; Salberg, M. M.; Zumbahlen, T. L.; Bain, G. A.; Liberta, A. E.; Valdés-Martínez, J.; Hernández-Ortega, S.; Toscano, R. A. Polyhedron 1995, 14, 2189-2200. [ Links ]
7. Valdés-Martínez, J.; Hernández-Ortega, S.; West, D.X.; Stark, A.M.; Bain, G.A. J. Chem Cryst. 1996, 26, 861-864. [ Links ]
8. Valdés-Martínez, J.; Hernández-Ortega, S.; West, D.X.; Ives, J.S.; Bain, G.A. Z. Krystallogr. 1998, 213, 246-248. [ Links ]
9. Etter, M. C. Acc. Chem. Res. 1990, 23, 120-126. [ Links ]
10. Taylor, R.; Kennard, O. J. Am. Chem. Soc. 1982, 104, 5063-5070. [ Links ]
11. West, D.X.; Ingram III, J. J.; Kozub, N. M.; Bain, G. A.; Liberta, A. E. Transition Met. Chem. 1996, 21, 213-218. [ Links ]














