Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Sociedad Química de México
versión impresa ISSN 0583-7693
Rev. Soc. Quím. Méx vol.44 no.2 Ciudad de México abr./jun. 2000
Investigación
Oxygen Measurement in Gas Mixtures
Héctor G. Riveros,1* Pilar Ovalle-Marroquín2 and Armando Lara1
1 Instituto de Física UNAM, Circuito de la Investigación Científica, Ciudad Universitaria. 04510 Coyoacán, México D. F. Tel.: 5622-5091, Fax: 5622-5008. e-mail: riveros@fenix.ifisicacu.unam.mx
2 Instituto de Química UNAM, Circuito de la Investigación Científica, Ciudad Universitaria. 04510 Coyoacán, México D. F.
Recibido el 18 de enero del 2000.
Aceptado el 22 de febrero del 2000.
Abstract
The measurement of oxygen y gaseous mixtures has many applications, and then a variety of sensors have been developed. Also, many books for children say wrongly, that they "measure" the oxygen in the air with a lit candle. We use the slow oxidation of iron, to absorb the oxygen in the air, and use the change in gas volume to measure its concentration, avoiding problems related to the combustion products and the heat released in the reaction.
Key Words: Oxygen, gas mixtures, oxidation, absorbtion, concentration, combustion products.
Resumen
La medida del oxígeno en mezclas gaseosas tiene muchas aplicaciones y se han desarrollado una gran variedad de sensores. Muchos libros para niños dicen equivocadamente, que lo miden con una vela encendida. Nosotros usamos la oxidación lenta del hierro para absorber el oxígeno del aire, usando el cambio en volumen para medir su concentración, evitando los problemas asociados a los productos de combustión y al calor liberado en la combustión.
Palabras clave: Oxígeno, mezcla gaseosa, oxidación, absorción, concentración, combustion.
A la memoria del Dr. Jacobo Gómez-Lara
Introduction
The measurement of oxygen in gaseous mixtures has many applications, especially in biology, to follow the oxygen consumption in living organisms. Then a variety of sensors have been developed: electromechanical sensors for the analysis of blood-gases, gases and vapors in clinical medicine [1], mass spectrometer for measuring fractional concentrations of oxygen and carbon dioxide [2], measurements with flow-through indirect colorimetry [3], nondispersive absorption [4], etc.
Those sensors have been used as metabolic monitors [5], in patients under general anesthesia [6], walking oxygen consumption rate [7], during exercise [8, 9], to test the hypothesis of the temperature-dependence of the kinetics of O2 consumption as muscle temperature decreases [10], oxygen consumption in emperor penguins [11], and the oxygen consumption and "suffocated point" of adults Penaeus japonicus (a shrimp) [12]. Neurotic patients were differed from healthy individuals by lower both absolute and relative maximal oxygen consumption (MOC) values as well as by lower indexes of lungs ventilations, CO2 elimination, respiration frequencies and breath depth. It was supposed that MOC as well energy potentials decrease may be of significant importance in neurotic disturbances development [13]. Also, to predict the rate of O2 consumption in a scaled nuclear fuel waste disposal vault as a result of container corrosion, reaction with biotite and the oxidation of organics and other oxidizable impurities in the clay [14].
Many books for children "measure" the oxygen in the air trapping some air with a lit candle over water. They think that the fast reaction of O2 with the paraffin (combustion) make it disappears, but neglect the gases produced in the combustion, and forget about the thermal effects of burning [15]. The heat from the candle expand the trapped gases, and the contraction on cooling at room temperature, could produce the 20 % reduction in gas volume interpreted as the "oxygen consumption". Other author [16] uses the question "How long can we breathe?" to introduce his students to the art of measuring, without measuring the level of oxygen in the air that produces the sensation of "suffocating".
In this paper we propose the slow oxidation of iron, to absorb the oxygen in the air to a solid form, and use the change in gas volume to measure its concentration. This procedure avoids the problems related to the combustion products and the heat released in the reaction. Also, we use the same procedure to measure the change in oxygen in breathed air, and how many times we can re-breath it, answering the problem proposed in the last reference.
The proposed reaction is:
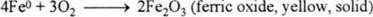
Other reactions can be used to absorb other gases in mixtures. The reaction rate should be proportional to the oxygen concentration and the reactive surface. If the reactive surface and temperature are constants, the oxygen concentration disappears exponentially with a time constant τ depending on reactive area and temperature.
Experimental
We use thin iron fiber, of the kind used to wash dishes, to lead the reaction in practical times. Because one is thinner than the other, we use thin and thick on the graphs. The fiber was washed with soap to degrease it and put about 1.25 grams of each expanded fiber in the bottom of 100 mL measuring cylinders. Filled with water, the cylinders were inverted on a water tank, trapping about 95 mL of air. A third measuring cylinder, only with air, was used as a reference for the change in volume associated to changes in room temperature. The data of temperature measurement with a thermistor were not used, because its correlation with the change in volume was poor. The change in volume was measured as a function of time as shown in Fig. 1. The amount of oxygen exhaled in each inhalation and the concentration when the first symptoms of suffocation occurs, were measured using the same procedure. The cylinders were filled with air breathed from 1 to 5 times. Using a plastic hose, an empty plastic bag was filled with expired air, inspiring and expiring the same air, were obtained samples for 1 to 5 respirations. After 5 times a feeling of suffocation occurs.

Results and Discussion
Figure 1 shows the measurement for each fiber, and adjusted exponential curves with time constants of 10 and 21 hours for the thin and thick fibers. This is a nice example of an exponential reaction and that the reaction rate depends on the reactive area, the thinner fiber should have about twice the area. Measuring at very short times, we may see the changes due to the draining and evaporation of the water trapped by capillarity on the iron fiber.
After 5 times of re-breathing the same air a feeling of suffocation occurs. Fig. 2 shows the absorption curve for air breathed 3 and 5 times, following the expected exponential trend but with an order of magnitude lower time constants. That means that the presence of carbon dioxide increases the reaction rate.
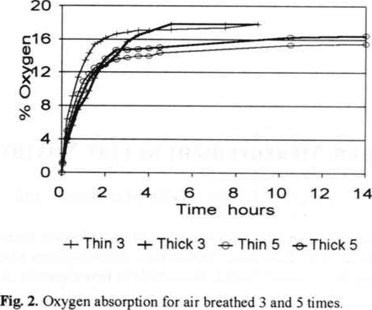
Fig. 3 shows the final oxygen concentration after 0 to 5 breathing procedures, for a single subject in a sedentary position, as measured using a thin or a thick iron fiber. The departure from a linear relationship could be attributed to lack of control on the rate of breathing, or the amount of time the air stay inside the lungs. But a reduction of 25% in the oxygen in the air, produces a sensation of suffocation giving a practical limit for the calculation of how long we can breath inside a closed room.

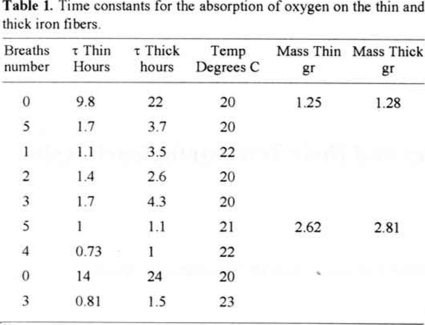
Another interesting result comes from the measurement of the time constants for the many trials. Table 1 shows the adjusted time constants in the order of the measurement. The initial measurements were made using the same iron fiber. Because of the oxidation reaction the fiber slowly disappears, the effective area decrease and the time constant increases. Whe the amount of iron fibber was changed to about twice, the next measurements shows the expected reduction in time constant associated to the duplication of the effective area, proportional to the iron mass.
Conclusions
1. Using the oxidation of iron in air we can measure the amount of oxygen present in the mixture.
2. It provides a nice example of an exponential reaction.
3. The time constant is affected by the previous treatment of the iron fibers.
4. A reduction of 25% in the oxygen concentration in the air produces the sensation of suffocation.
5. The time constant for the oxidation reaction are very different for dry fiber, wet fiber or in the presence of carbon dioxide.
References
1. Hahn, C.E. Analyst 1998, 123, 57R. [ Links ]
2. Sharma, C.; Gallagher, R.R. Biomed. Sci. Instrum. 1994, 30, 1. [ Links ]
3. Bauer, K.; Pasel, K.;Uhrig, C.; Sperling, P.; Versmold, H. Pediatr. Res. 1997, 41, 139-144. [ Links ]
4. Arnoudse, P.B.; Pardue, H.L.; Bourland, J.D.; Miller, R. ; Geddes, L.A. Anal. Chem. 1992, 64, 200-203. [ Links ]
5. Fernando, T.L.; Packer, J.S.; Cade, J.F. Control Engineering Practice 1995, 3, 1433-1440. [ Links ]
6. Barnard, J.P.; Sleigh, J.W. Br. J. Anaesth. 1995, 74, 155-158. [ Links ]
7. Prediction of oxygen consumption rates from heart interval mean and variance. IEEE. Anonymous 1997, 725-726.
8. Peel, C. ; Utsey, C. Med. Sci. Sports Exerc. 1993, 25, 396-400. [ Links ]
9. Zoladz, J.A.; Duda, K.;Majerczak, J. Eur. J. Appl. Physiol. 1998, 77, 445-451. [ Links ]
10. Ferretti, G.; Binzoni, T.; Hulo, N.; Kayser, B.; Thomet, J.M. ; Cerretelli, P. Respir. Physiol. 1995, 102, 261-8. [ Links ]
11. Kooyman, G.L.; Ponganis, P.J. Journal of Experimental Biology 1994, 195, 199-209. [ Links ]
12. Dai, Q.; Wang, J.; Li, Q. Journal of Xiamen Fisheries College/Xiamen Shuichan Xueyuan Xuebao. Xiamen 1994, 16, 25-29. [ Links ]
13. Gorozhanin, V.S.; Maksimova, M.I. Zh. Nevropatol. Psikhiatr. Im. S S Korsakova 1995, 95, 48-51. [ Links ]
14. Murphy, W.M.; Knecht, D.A. Mater. Res. Soc. 1996, 547-554. [ Links ]
15. Caplan, J.B.; Gerritsen, H.J.; LeDell J.S. The Physics Teacher, 1994, 32, 310-314. [ Links ]
16. Larrabee, DA. The Physics Teacher 1998, 36, 36-38. [ Links ]














