Serviços Personalizados
Journal
Artigo
Indicadores
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Agricultura técnica en México
versão impressa ISSN 0568-2517
Agric. Téc. Méx vol.34 no.4 México Out./Dez. 2008
Artículos
Responses of potato cultivars to the psyllid (Bactericera cockerelli) under greenhouse conditions*
Respuesta de variedades de papa al psílido (Bactericera cockerelli) bajo condiciones de invernadero
Margarita Díaz–Valasis1, Mateo Armando Cadena–Hinojosa1§, Reyna Isabel Rojas Martínez2, Emma Zavaleta–Mejía2, Daniel Ochoa Martínez2 y Rafael Bujanos Muñiz3
1 Campo Experimental Valle de México, INIFAP, A. P. 10, km 18.5 carretera Los Reyes–Lechería, Texcoco, Estado de México C. P. 56230.
2 Posgrado en Fitosanidad, Colegio de Postgraduados, km 35.5 carretera México Texcoco, Montecillo, Estado de México C. P. 56230.
3 Campo Experimental Bajío, INIFAP, A. P. 112, km 6.5 carretera Celaya–San Miguel de Allende, Celaya, Guanajuato, México C. P. 38110.
§ Corresponding author:
machgg2@yahoo.com.mx
*Recibido: Enero de 2008
Aceptado: Diciembre de 2008
ABSTRACT
The responses of 20 potato cultivars to the psyllid Bactericera cockerelli (= Paratrioza cockerelli) were studied under greenhouse conditions. Two different trials were conducted, in one two months after planting, 200 adult insects, taken from a phytoplasms–free psyllid colony checked by PCR technique, were released inside a cage in which the potato plants were growing. No insects were placed in a control cage. At the end of the growth cycle following traits were recorded: number of nymphs plant–1, tuber yield (g plant–1) and number of tubers plant–1, also records were made of the degree of internal tuber browning and any abnormal tuber sprouting. In a second trial, psyllid yellows and its capacity to be transmitted in tubers and through grafts were studied in two susceptible cultivars. In the first trial all genotypes showed typical yellowing symptoms on foliage after 15–20 days of exposure to the insect. The number of nymphs among cultivars varied from 90 to 450 plant–1 in 2004 and from 490 to 1800 in 2005. Cultivars Alpha, Gigant, NAU–6 and Lady Rosetta were tolerant since they showed from none to mild internal tuber browning caused by the psyllid. In the rest of the cultivars, discoloration varied from moderate to strong. Secondary tuber transmission and serial transmission of psyllid yellows by grafting were not observed; neither foliar symptoms nor internal browning were detected, and sprouting was normal. In both years, yield and number of tubers were significantly reduced by B. cockerelli in all cultivars. Internal browning, premature sprouting, lack of sprouting, and hairy sprouts were all induced by the psyllid under greenhouse conditions in the absence of the phytoplasms associated with purple top disease.
Key words: paratrioza cockerelli, jumping lice, psyllid yellows, "salerillo".
RESUMEN
Se estudió en invernadero la respuesta de 20 variedades de papa al psílido Bactericera cockerelli (= Paratrioza cockerelli). Dos meses después de la siembra, 200 insectos adultos procedentes de una colonia libre de fitoplasmas, verificados por PCR, se liberaron dentro de una jaula conteniendo las plantas de papa, y ningún insecto en una segunda jaula de testigos. Al finalizar el ciclo de cultivo se registraron las características: número de ninfas, rendimiento y número de tubérculos planta–1, pardeamiento interno y brotación anormal de los tubérculos. En un segundo ensayo se determinó el amarillamiento por psílidos y la transmisión por tubérculo e injerto, en dos variedades susceptibles. Todos los genotipos mostraron síntomas típicos de amarillamiento foliar después de 15–20 días de exposición al insecto. El número de ninfas varió de 90 a 45 0 planta–1 en 2004, y de 490 a 1800 en 2005. Sólo Alpha, Gigant, NAU–6 y Lady Rosetta mostraron de ninguno a leve pardeamiento interno del tubérculo causado por el psílido y se consideran tolerantes. En el resto de las variedades este varió de moderado a fuerte. No se observó transmisión secundaria por tubérculo, ni la transmisión seriada por inj erto del amarillamiento; tampoco síntomas foliares, ni pardeamiento interno y la brotación fue normal. En ambos años el rendimiento y el número de tubérculos se redujeron significativamente en todas las variedades por B. cockerelli. El pardeamiento interno, brotación prematura o ausencia de la misma y brotes finos fueron inducidos por el psílido bajo condiciones de invernadero en ausencia de fitoplasmas asociados con la enfermedad de la punta morada de la papa.
Palabras clave: paratrioza cockerelli, amarillamiento por psílido, pulgón saltador, "salerillo".
INTRODUCTION
Tuber internal browning has been associated with the potato and tomato psyllid Bactericera cockerelli Sulc., a pest that is found in the main potato producing areas of Mexico and is thought to induce a metabolic disorder in the plant causing a syndrome in which early sprouting of tubers in colonized plants may occur. In western United States of America, this psyllid has been considered to be one of the most important insects pests of the potato crop (Cranshaw, 1993, 1994, 2002). Damage by B. cockerelli has been reported in the main potato producing areas of Mexico (Fuentes del Valle et al., 1960; Cranshaw, 1994; López Flores, 2002; Zavala Quintana, 2002) and is estimated that 70% of the area planted with potatoes is affected by the insect (Rubio–Covarrubias et al., 1997). Severe damage in tomato crops in California, USA, and in Baja California, Mexico has also been reported (Cranshaw, 1994). The psyllid can attack several crops. In comparison to other solanaceous crops, the potato is its favorite oviposition host (Pletsch, 1947). The symptoms of "psyllid yellows" in potato are retarded growth, erect growth and mild chlorosis in new foliage with basal cupping of leaves, and a progression of red coloration in new leaves. As the infestation by the insect progresses, upward rolling of leaves occurs throughout the plant, shortened and thickened terminal internodes result in rosettes, enlarged nodes, aerial tubers, or development of axillary branches, chlorosis of increasing intensity, plant growth may be at a standstill for weeks or up to a month, and premature senescence and death of plants occurs (Carter, 1962). In infested plants, symptoms are induced by nymphs (Richards, 1931) but not all nymphs are able to induce a toxic reaction (Cranshaw, 1993, 1994). Abnormal tuber sprouting (premature sprouting and weak sprouts) was observed in field grown potatoes (Cranshaw, 1994; 2002). The tubers showed internal browning and sometimes necrosis as well as hairy sprouts and occasionally did not sprout (Díaz–Valasis and Cadena–Hinojosa, unpublished data).
In 2004, the zebra chip effect, associated with B. cockerelli, was first reported. This disorder is characterized by symptoms that develop in potato tubers from infected plants and consists of a striped pattern of necrosis in tuber cross–sections; the necrosis becomes prominent when chips from infected tubers are fried. It was first observed in the southern United States of America, Mexico and Guatemala (Secor and Rivera–Varas, 2004).Also Munyaneza et al. (2007) reported the association of B. cockerelli with zebra chip symptoms, but did not mention tuber sprouting. Foliar and tuber symptoms induced by the insect are similar to those caused by potato purple top disease (PPT). However, damage caused by the psyllid is partially reversible (Cranshaw, 1994, 2002), while the symptoms induced by PPT are not (Cadena–Hinoj osa, 1974). The prevalence of PPT and the physiological disorders caused by B. cockerelli have been increasing in the central region of Mexico. In central Mexico, internal browning of tubers associated with PPT and B. cockerelli ranged from low to moderate in cultivars NAU–6, Alpha and Gigant, and from medium to medium–high in other cultivars. Abnormal sprouting symptoms ranged from lack of sprouts to the occurrence of weak ones. Hairy sprouts were commonly observed in the cultivars NAU–6, Alpha and Gigant. In cv. Norteña, no sprouting was detected (Cadena–Hinojosa et al., 2003). In spite of all these studies, the independent effect of the psyllid upon potato genotypes is still unknown. The present study was conducted to determine: 1) the effect of B. cockerelli on browning, sprouting, yield, number of tubers and foliar symptoms on potato plants in the absence of phytoplasmas, 2) to identify tolerant cultivars, and 3) to find out if the symptoms induced by psyllids in tubers are transmitted to future vegetative generations throughout grafting and tuber transmission.
MATERIALS AND METHODS
Establishment of a phytoplasms free B. cockerelli colony. During July and August 2003 nymphs at various development stages were collected from potato cultivars grown at Texcoco, State of Mexico. Initially, the nymphs were placed on potato plants kept in individual cages (foam cups covered with net) under a 14 h light period chamber at a temperature ranging from 10 to 25 °C. To increase the number of insects, a second colony was initiated from the potato one, on pepper plants of the Criollo Mixquic (Guajillo, Pulla) cultivar, on which B. cockerelli readily reproduces. During the development and reproduction of the colony, temperature and relative humidity in the cages were recorded with a HOBO data logger, model H–08–032–08#333699. Data were captured with Box Car Pro 3.51 software.
The mean daily temperature ± standard deviation (sd) (°C) was 16.5 ± 2.5, with a minimum mean of 7.4 ± 3.6 and a maximum mean of 25.5 ± 5.4. The mean and sd of recorded daily relative humidity (%) were: minimum 38.4 ± 27.5, mean 65.1 ± 18.3, maximum 91.7 ± 13.7, respectively.
Detection of phytoplasmas in the insect colony and host plants by PCR. In order to assess the presence or absence of phytoplasmas in the colony and host plants, the test was run before, during and at the end of the assay. Samples were taken at different developmental stages of the insect (egg, nymph and adult), as well as mixed samples of pepper plants where the colony was increased. Potato plants infested with the insect were also sampled by PCR assay in the 2004 and 2005 trials. DNA was extracted according to Ahrens and Seemuller (1992). PCR was run directly with the primer pair P1/Tint (Smart et al, 1996) that amplifies a 1600 bp fragment. Sequential nested PCR was used with P1/Tint as the first primer pair, followed by the universal primer R16F2/ R16R2 (Gundersen and Lee, 1996).
For the sequential PCR 1:30 dilutions of the first amplification cycle were made. A 1.0 uL aliquot of the dilution was used as DNA template in a total reaction volume of 25 uL that included: 50 ng of DNA, 0.5 uM of each initiator, 200 uM dNTPs (A, T, C, G), 2.0 mM MgCl2 and 2.5 DNA polymerase units (Amplificasa, Biotecnologias Universitarias, Mexico, D. F.). PCR reactions were performed in a Perkin Elmer model 2400 thermocy cler, with one denaturation cycle at 94 °C for 2 min and 35 additional cycles of 1 min of denaturation at 94 °C, 1 min of annealing at 55 °C and 1.5 min of polymerization at 72 °C. The final extension phase was at 72 °C for 7 min. The PCR products were fractionated in 1.5% agarose gels, dyed with ethidium bromide (0.5 μgmL–1) and observed under uv light.
Responses of potato cultivars exposed to B. cockerelli. In the summer of 2004 and 2005, 20 potato cultivars from greenhouse–grown tubers of Alpha, Atlantic, Bintje, Gigant, Granola, Lady Rosetta, Lupita, Marciana, Malinche, Michoacán, Mondial, Montserrat, NAU–6, Norteña, Sancal, Sangema, San Jose, Zafiro, 4–11 and 676008–Roja, that had been visually checked and with ELISA techniques for the main potato viruses, were established in cages (0.70 m high, 2.72 m long and 1.30 m wide) and kept in the greenhouse. The experiment was set out with four replicates of each genotype and a second set of plants as controls (the same cultivars without insects in separate cages). Two months after planting, 200 adult insects were released into one of the cage s (5 0 insects in each quarter of the cage). The psyllids were obtained from the previously established pepper colony and were free of phytoplasms according to the PCR test. Foliar symptoms were observed at weekly intervals, and temperature and relative humidity were recorded every hour with a HOBO data logger.
At the end of the growth cycle (average 105 days) 10 leaves per plant were sampled and the number of nymphs per cultivar counted. The counting was done under a stereo–microscope to aid detection of the early nymphal stages of the insect. On all cultivars following traits were recorded at harvest: tuber yield (g plant–1), number of tubers per plant and degree of tuber browning. To evaluate browning, the following scale was used: 1= no browning, 2= very mild, 3= mild, 4= mild–moderate, 5= moderate, 6= moderate–strong, 7= strong. Tuber sprouting was observed 2–3 months after harvest.
Statistical analysis. Although due to the nature of the assay we were unable to adjust to an experimental design, for the statistical analysis, a completely randomized block design was implemented. Yield and number of tubers per plant for plants with or without insects, and nymphs plant–1 in 2004 and 2005 were subjected to analysis of variance. When significant differences were found, a Tukey test at a p= 0.05 was performed.
Secondary seed tuber transmission of the effect of psyllid yellows in the greenhouse. To determine if abnormal symptoms of yellowing, browning and sprouting induced by the potato psyllid were present in the next generation of plants, tubers with hairy sprouts were planted in pots with sterilized peat moss. Foliage readings were taken during 90 days after the emergency of plants. Tubers were checked for browning 30 days after the last foliage reading and sprouting was observed 3–5 months once it had started.
Graft transmission from plants with yellowing symptoms into healthy potato plants. Serial grafts were made in order to determine up to how many generations psyllid yellows could be transmitted, and to test whether these symptoms were really induced only by the insect and not by an infectious agent. Branches with leaves were taken from plants with typical symptoms and grafted on six plants of the susceptible cultivars Norteña and Michoacán. Weekly observations were made looking for foliar symptoms. One month after grafting, new grafts were taken to insert on new plants of the same cultivar. Tubers were harvested and new assessments of browning were made from both sets of plants.
RESULTS
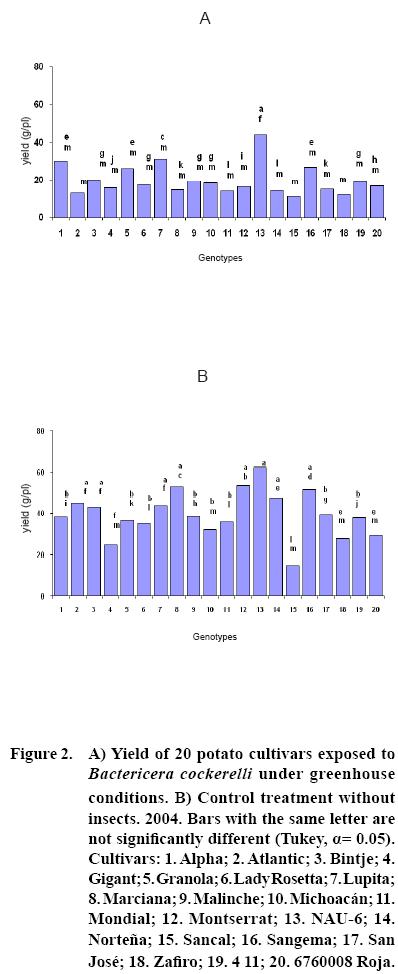
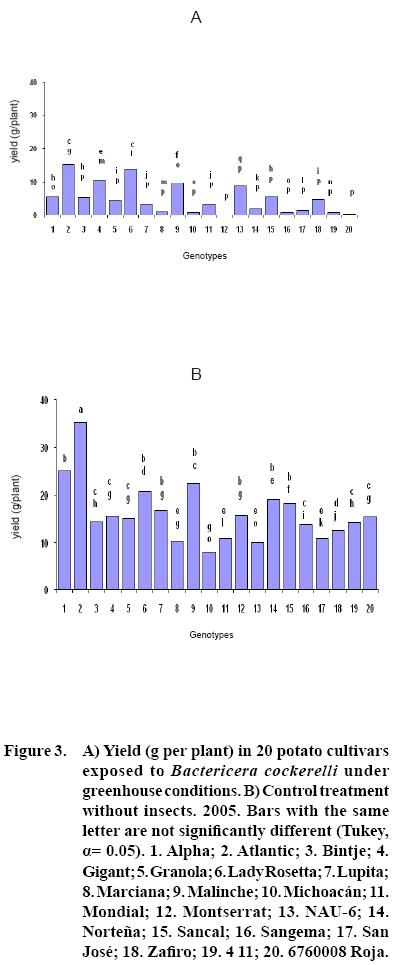
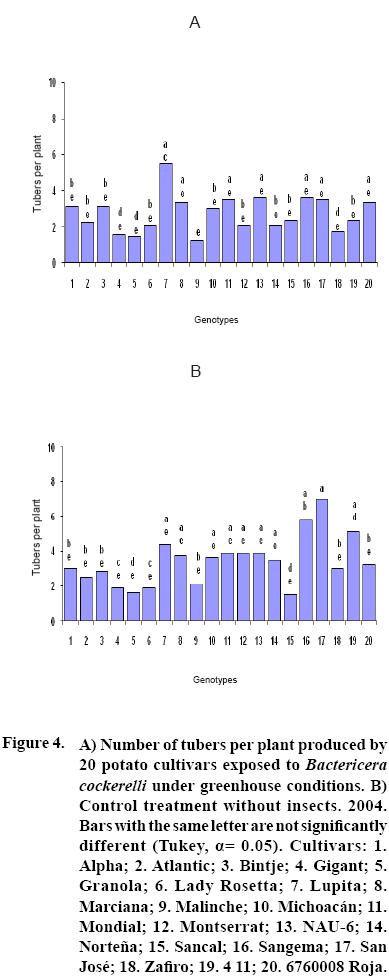
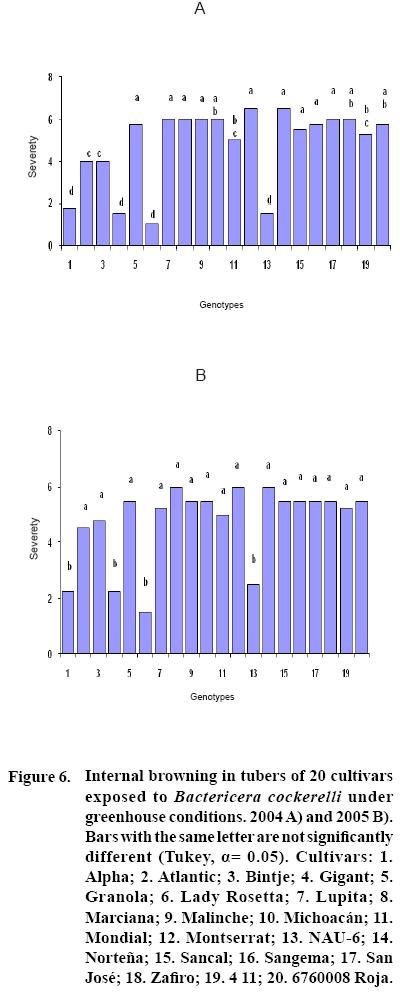
After 15 to 20 days of exposure to the Insect, all cultivars showed chlorosis and typical yellowing symptoms on the foliage. PCR tests to detect phytoplasms in the symptomatic foliage and in the insects (adults and nymphs) were negative. By the end of the growth cycle from 90 to 450 nymphs per plant were found in 2004 and 490 to 1800 in 2005 (Figure 1). Yield of plants exposed to the insect were significantly lower (p<0.05) than those in the controls in both years (Figures 2, 3). Average yield was reduced by 49.4% in 2004 and 70.0% in 2005. In both years tuber number was also reduced, 19.2% in 2004 and 70.0% in 2005, but only in 2005 the reduction was significant (p<0.05) (Figures 4, 5). All cultivars showed similar response in terms of tuber browning during the two years of evaluation. The small yearly differences in intensity resulted in consistent readings between the two years (Figure 6). Lady Rosetta showed nil to mild browning, followed by Alpha, Gigant, and NAU–6. Atlantic and Bintje displayed mild moderate to moderate browning; Mondial had moderate browning; Granola, Lupita, Malinche, Michoacán, Sancal, Sangema, San José, Zafiro, 4–11 and 676008 Roja showed moderate to moderately strong browning; and Marciana, Montserrat and Norteña displayed severe symptoms that varied from moderately strong to strong browning.
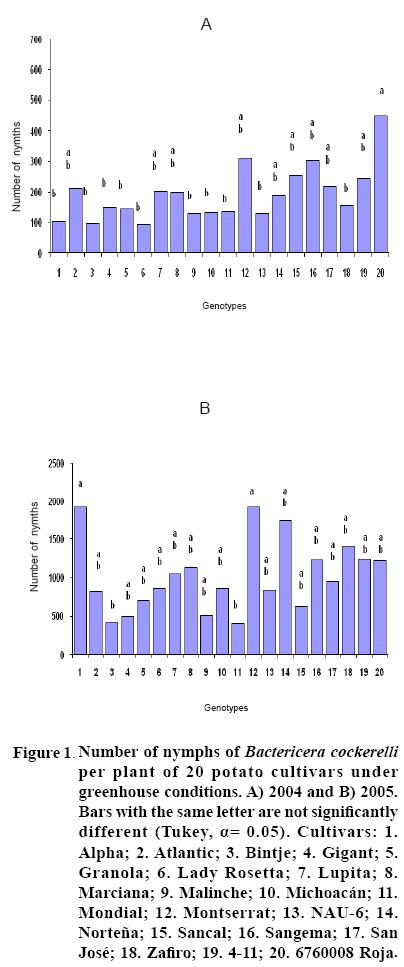
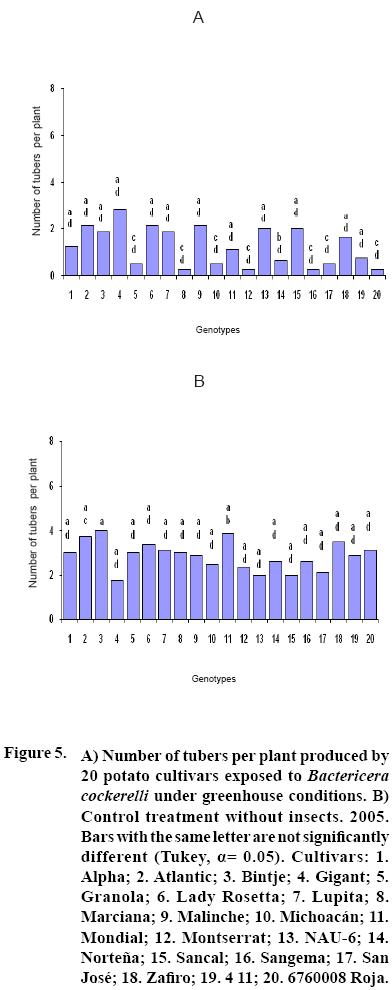
In both years tubers from all cultivars exposed to the insect displayed abnormal sprouting. Different types of abnormal sprouting were recorded: hairy sprouts as in cultivars Gigant, Lady Rosetta and NAU–6, and lack of sprouts in Michoacán, Montserrat, Sangema and Zafiro. Plants developed from the tubers of plants exposed to the insect displayed retarded growth and less vigor as compared to controls, but did not show psyllid induced yellowing during their development. Tubers produced from all cultivars in the subsequent generation sprouted normally and showed no browning.
Graft transmission from plants with yellowing symptoms on healthy potato plants. Only four out of 16 grafted Norteña plants developed psyllid yellows symptoms and when the same cultivar was grafted again there were no defined or consistent symptoms in the grafted plants. Cultivar Michoacán showed no symptoms. There was no browning in the tubers from the two tested cultivars and sprouting was normal.
DISCUSSION
As previously reported (Carter, 1962), foliar symptoms were evident after 15 to 20 days of exposure to the psyllids. The psyllids reduced yield, tuber size, and induced abnormal sprouting. Cultivars Atlantic, Bintje, Gigant, Granola, Lady Rosetta, Malinche, Mondial and NAU–6 showed abnormal premature sprouting in both years, perhaps caused by the disruption of dormancy induced by the psyllid (Carter, 1962). Production of chain tubers, as reported by these researchers, was not observed. It is possible that that a particular symptom was not produced by the insect's presence, but also by the environmental conditions, the influence of other pathogen (e.g. purple top), the specific response of the genotypes, or a combination of these or other factors.
In this research, cultivars Alpha, Gigant, and NAU–6 showed none or only mild browning, as in previously reported (Cadena–Hinojosa et al., 2003). Even though these cultivars showed from none to only mild internal browning in the tubers, they did not show complete resistance to the psyllid since all four displayed foliar symptoms; nevertheless, they could be used as a resistance source in a potato breeding program. Cultivars Granola, Lupita, Malinche, Michoacan, Sancal, Sangema, San Jose, Zafiro, 4–11 and 676008 Roja showed from moderate to strong moderate browning in our study; Marciana, Michoacán, Montserrat, Norteña and Zafiro displayed severe browning that fluctuated from moderate–strong to strong, comparable to the degree of severity they have shown in the field (Cadena–Hinojosa et al., 2003).
In the present study, most of the evaluated cultivars showed internal browning and hairy sprout, and there were four with nil to mild browning that displayed hairy sprouts under stored conditions. Recently, Munyanesa et al. (2007) reported the association of B. cockerelli with zebra chip symptoms in tubers, which consists of a striped pattern made more evident after frying, but did not mention tuber sprouting. The browning observed in the tubers before frying is different from the symptoms we found in several cultivars i.e. Lady Rosetta, NAU–6, and Gigant.
With regard to seed transmission of psyllid yellows, plants grown from tubers from symptomatic plants were slow to develop initially and lacked vigor compared to the controls. However, no psyllid yellows symptoms were observed during the growth of these plants; no internal browning was detected and tuber sprouting was normal in the cultivars tested. This suggests a gradual recovery of the plants through generations. This supports the idea that this disorder may not be infectious and it probably involves one or more toxins (Abernathy, 1991). The concentration of those toxins in the plant tissue diminishes through time, allowing the recovery of the plant and the remission of the initial symptoms. Even though each of our test plants was grafted with three shoots, which came from plants showing clear symptoms and presumably containing high concentrations of the phytotoxic principle(s), only a low percentage of graft transmission (25%) of psyllid yellows symptoms was observed.
When tissue grafts did result in symptom development and further grafts were then made to plants of the same cultivar, no clear or consistent symptoms were observed and neither did tubers show internal browning. These results agree with work reported by others indicating that tissue taken from plants heavily loaded with the psyllid toxin will carry over the phytotoxic principle in sufficient amounts to cause psyllid yellows in healthy plants, but that subsequent grafts show a gradual recovery in the form of a reversible reaction (Cranshaw, 1993). This strengthens the idea that metabolites produced by the psyllid are responsible for the induced symptoms.
CONCLUSIONS
In both years, all cultivars displayed foliar symptoms in response to B. cockerelli and yield and number of tubers was significantly reduced.
Internal browning of tubers, premature sprouting, hairy sprouts and lack of sprouting were all induced by the psyllid under greenhouse conditions and in the absence of phytoplasms associated to the purple top disease.
Cultivars NAU–6, Alpha, Gigant and Lady Rosetta were resistant to internal tuber browning, and could be used as sources of resistance.
Psyllid symptoms induced in tubers were not transmitted to a subsequent generation by either tubers or grafting.
LITERATURE CITED
Abernathy, R. L. 1991. Investigations into the nature of the potato psyllid toxin. M. S. thesis. Colorado State University, Fort Collins, CO. 54 p. [ Links ]
Ahrens, U. and Seemuller, E. 1992. Detection of DNA of plant pathogenic mycoplasma–like organisms by a polymerase chain reaction that amplifies a sequence of the 16S rRNA gene. Phytopathol. 82:828–832. [ Links ]
Cadena–Hinojosa, M. A. 1974. Estudios sobre la "Punta Morada" de la papa. Tesis de Maestría. Centro de Fitopatología, Colegio de Postgraduados, Chapingo, Estado de México. 70 p. [ Links ]
Cadena–Hinojosa, M. A.; Guzmán–Plazola, R.; Díaz–Valasis, M.; Zavala–Quintana, T. E.; Magaña–Torres, O. S.; Almeyda–León, I. H.; López–Delgado, H., Rivera–Peña, A. y Rubio–Covarrubias, O. 2003. Distribución, incidencia y severidad del pardeamiento y la brotación anormal en los tubérculos de papa (Solanum tuberosum L.) en Valles Altos y sierras de los estados de México, Tlaxcala y el Distrito Federal, México. Rev. Mex. Fitopat. 21:248–259. [ Links ]
Carter, W. 1962. Insects in Relation to Plant Diseases. Interscience Pub. New York. 705 p. [ Links ]
Cranshaw, W. S. 1993. An annotated bibliography of potato/tomato psyllid, Paratrioza cockerelli (Sulc.) (Homoptera: Psyllidae). Colorado State University, Agricultural Experiment Station Bulletin TB93–5. Ft. Collins, CO. 52 p. [ Links ]
Cranshaw, W. S. 1994. The potato (tomato) psyllid, Paratrioza cockerelli (Sulc.) as a pest of potatoes). In: Advances in Potato Pest Biology and Management, G. W. Szender, L. M. Powelson, R. K. Jansson, and K. V. Raman (eds). The American Phytopathological Society. St. Paul, MN. p. 83–94. [ Links ]
Cranshaw, W. S. 2002. Manejo del psílido de la papa/tomate en el cultivo de la papa. Memorias del XI Congreso Nacional de Productores de Papa. Septiembre 26–28, 2002. León, Guanajuato, México. p. 46–51. [ Links ]
Fuentes del Valle, O.; García Palacios, O. y Mercado Guerrero, A. 1960. Control de plagas y del amarillamiento de la papa con Thimet al 10% granulado en la región de Navidad Nuevo León. II Congreso Nacional de Entomología y Fitopatología. Escuela Nacional de Agricultura. Chapingo, Estado de México. p. 199–205. [ Links ]
Gundersen, D. E. and Lee, I. M. 1996. Ultrasensitive detection of phytoplasmas by nested–PCR assays using two universal primer pairs. Phytopathol. Medit. 35:144–151. [ Links ]
López Flores, C. I. 2002. Estudios sobre el psílido de la papa (Paratrioza cockerelli) en la región del Bajío. Memorias del XI Congreso Nacional de Productores de Papa. Septiembre 26–28, 2002. León, Guanajuato, México. p. 98–109. [ Links ]
Munyaneza, J. E.; Crosslin, J. M. and Upton, J. E. 2007. Association of Bactericera cockerelli (Homoptera: Psyllidae) with" Zebra Chip," a new potato disease in southwestern united States and Mexico. J. Econ. Entomol. 100:656–663. [ Links ]
Pletsch, D. J. 1947. The potato psyllid Paratrioza cockerelli (Sulc.), its biology and control. Montana Agricultural Experiment Station Bulletin 446, 95 p. [ Links ]
Richards, B. L. 1931. Further studies with psyllid yellows of the potato. Phytopathol. 21:103 (Abstract). [ Links ]
Rubio–Covarrubias, O. A.; Flores, G. F. X.; Borbón, S. J. T.; Cadena–Hinojosa, M. A.; Díaz, H. C.; Flores, L. R.; González, H. A.; Guevara, L. J.; Hernández, J. S.; Magallanes, G. J. V.; Paredes, T. A.; Parga, T. V. M.; Rivera, P. A.; Rocha, R. R. y Zavala, Q. T. E. 1997. Programa nacional de investigación en el cultivo de Papa. INIFAP. México, D. F. 61 p. Publicación Especial No. 13. [ Links ]
Sanford, G. B. 1952. Phloem necrosis of potato tubers associated with infestation of vines by Paratrioza cockerelli (Sulc.). Sci. Agr. 32:433–439. [ Links ]
Secor, G. A. and Rivera–Varas, V. V. 2004. Emerging diseases of cultivated potato and their impact on Latin America. Rev. Latinoam. de la Papa (Suppl.) 1:1–8. [ Links ]
Smart, C. D.; Schneider, B.; Bomquist, C. L.; Guerra, L. J.; Harrison, N. A.; Ahrens, U.; Lorenz, K. H.; Seemüller, E. and Kirkpatrick, B. C. 1996. Phytoplasma–specific PCR primers based on sequences of the 16–23S rRNA spacer region. App. and Environm. Microbiol. 62:2988–2993. [ Links ]
Snyder, W. C.; Thomas, H. E.; and Fairchild, S. J. 1946a. A type of internal necrosis of the potato tuber caused by psyllids. Phytopathol. 36:480–481. [ Links ]
Snyder, W. C.; Thomas, H. E.; and Fairchild , S. J. 1946b. Spindling or hair sprout of potato. Phytopathol. 36:897–904. [ Links ]
Zavala–Quintana, T. E. 2002. Experiencias en el Valle de Toluca sobre Punta Morada. Memorias del XII Congreso Nacional de Productores de Papa. Septiembre 2002. León Guanajuato, México. p. 81–97. [ Links ]














