Tabla: Highlights
| • Ketamine has been shown to rapidly improve depression and has been promoted in young adults. |
| • Ketamine in older population has not been widely studied. |
| • Single infusion of ketamine could improve depressive symptoms in elderly patients. |
| • Administration of low dosages (0.5 mg/kg) of ketamine was associated with good tolerability profile in elderly patients. |
| • This work provides useful information regarding novelty evidence for at least a short-term effect of ketamine on depressive symptom severity in a minor-sedative surgical context. |
Introduction
The aging population worldwide increases the occurrence of different age-related conditions, such as chronic diseases and mental health issues, such as late-life depressive symptoms1. Depressive disorder is the most prevalent mental illness worldwide and has the second-highest morbidity rate worldwide2,3. In elderly adults, the prevalence of clinical depression ranges from 4 to 30%4-6. It is associated with a lower quality of life7, more significant medical comorbidity, increased costs and use of medical services, and increased mortality rates8,9.
Several options are available to treat mild to severe depression in the elderly. Although they are effective and well-tolerated drugs10, most of them have a profile of adverse effects associated with suboptimal adherence11. In addition, it takes several weeks to observe a significant clinical impact. Only 50% of patients achieve remission, and up to 30% do not respond to treatment12,13. Although alternative treatments such as electroconvulsive therapy are associated with a short response interval, their association with cardiovascular complications limits their use in this population14. Therefore, further research is required to develop more efficient, safe, and rapidly acting treatment options15,16. Recently, the use of ketamine for treatment-resistant depression has been examined. Among other substances, ketamine has been shown to be useful and effective against depressive symptoms, even after only one administration. Ketamine acts at different levels because it is a derivative of arylcyclohexylamine and a noncompetitive antagonist of the N-methyl-D-aspartate (NMDA) receptor. It produces effects such as sedation, analgesia, and anesthesia17. Although it has been used in anesthesia for more than 40 years18, in the last decade, there has been an increasing interest in ketamine as an alternative treatment for affective disorders, especially for treatment-resistant depression19. Ketamine use is associated with a rapid antidepressant effect20,21. A single dose of ketamine produces a rapid response and up to a week of reduced depressive symptoms compared with placebo22,23. Multiple controlled clinical trials have demonstrated the efficacy of this alternative treatment for major depressive disorder. Nevertheless, studies with elderly populations are scarce; therefore, the safety profile of this treatment and its effects on depressive symptoms remain unclear24,25. We aimed to examine the effect, safety profile, and feasibility of a possible novel intervention in a larger scale study of a single infusion of 0.5 mg/kg ketamine on depressive symptoms in elderly patients.
Material and methods
The study protocol and procedures were approved by the Medical Center Institutional Review Board (IRB# R-3601-148) of Centro Médico Nacional Siglo XXI, which belongs to the Mexican Social Security Institute (IMSS, in Spanish). The trial was registered before patient enrollment at clinicaltrials.gov (NCT347431; principal investigator: Rascón Martínez D.M.; date of registration: March 22, 2018). All participants signed an informed consent form, and the study followed the guidelines provided in the declaration of Helsinki.
Due to the severity of visual disability and taking advantage of the monitoring and conscious sedation technique adding ketamine as an option for ophthalmological sedation procedures, all patients who required ophthalmological surgery using retrobulbar block were invited to participate. The following inclusion criteria were met: 1) men and women aged 60 years or above; 2) intraocular pressure of < 20 mmHg; and 3) American Society of Anesthesiologists Physical Status classification (ASA-PS) from I to III. The following were the exclusion criteria: 1) previous antidepressant medications; 2) moderate cognitive impairment according to the Short Portable Mental Status Questionnaire assessment (SPMSQ)26; 3) a record of mental illnesses in their clinical file; 4) nephropathy; 5) history of difficulties in controlling arterial blood pressure, uncontrolled hepatic disorders, or adverse response to ketamine, as per clinical records.
Patients who presented postoperative delirium diagnosed by the Confusion Assessment Method (CAM)27 were also not included in the analysis.
Study design and procedures. This was a randomized, double-blind, dose-controlled comparative study. The purpose of the present study was to examine the feasibility and tolerability of ketamine for a possible novel intervention in a larger-scale study28.
A randomization schedule was created using random number Tables generated from the SPSS program and concealed these results in sequentially numbered envelopes. According to this, nurses unrelated to the research project open the sealed envelopes and prepare the medications to be administered by blinded anesthesiologists before each case.
In the experimental group, ketamine was administered at a 0.5 mg/kg dose in NaCl solution at 0.9% (250 mL), whereas in the control group, only NaCl solution at 0.9% with the same physical characteristics of the ketamine solution was administered. Infusion velocity was calculated using the routine time for a particular type of procedure, usually no more than two hours throughout the surgery. The dosage of ketamine was based on previous studies, which utilized around 0.3-0.5 mg/kg of ketamine for depression29,30. Both groups induced initial mild sedation using midazolam 0.01 mg/kg + fentanyl 1 μg/kg. Once in the surgery room, patients were monitored with an electrocardiograph, pulse oximeter, sphygmomanometer, plethysmograph, and supplemented oxygen tips.
To evaluate the presence of depressive symptoms and the effect of the administration of ketamine, the Spanish version of the 15 item GDS-SF31 was applied at three intervals: at the time of hospitalization, 120 min after surgery, and 24 hours after surgery. The GDS-SF is one of the most frequently used instruments to assess depression in the elderly and has shown adequate reliability and validity values in the Mexican population. The scale comprises 15 items of «yes/no» answer with a score ranging from 0 to 15, with a score of 5 indicating mild depression. Although it is a self-reported instrument, it was applied by trained personnel due to the visual deficiencies of the patients. For baseline evaluation, the participants were questioned regarding the presence of symptoms during the previous week, and for the follow-up evaluation, the interviews focused on the participants’ state in the last hours. This procedure has been previously performed using instruments to evaluate depressive symptoms in interventions associated with early response.
In addition, we evaluated sedation using the Ramsay test32, where a range from two to four was required, which means the patients can be sleepy under sedative effects but oriented, cooperative, and responding commands through surgery and when patients are ready for discharge. Also, during this time, we recorded the vital signs as well as any indicative psychotomimetic effects as nystagmus, respiratory depression, or hallucinations. Since these effects are expected no more than 45 minutes (half-life elimination) once the administration of ketamine is stopped, we asked patients every 30 minutes about general conditions during ketamine infusion and before discharge. Finally, the infusion was suspended during the study for any reason considered a risk to the patient, like difficulty in controlling blood pressure and arrhythmia or requirement of any additional medication other than the study medications.
Due to the lack of previous studies assessing depressive symptoms in patients during ophthalmological surgery with the use of ketamine, we decided to calculate sample size in accordance with an a priori specified response criteria based on a ≥ 50% decrease in depressive symptom severity33 from baseline depressive symptom severity to study endpoint. With a 90% power and 5% alpha error, 39 patients were selected for each treatment group. Moreover, we arbitrarily chose to test at least 10 additional subjects per group, considering some might not complete the study.
Statistical analysis. Statistical analyses were performed with IBM SPSS Statistics, version 20 (IBM, Armonk, NY). Normal distribution of the variables was tested with asymmetry and kurtosis, with values found between an acceptable range (from 0.85 to 2.1 for asymmetry and from 0.50 to 0.53 for kurtosis). Descriptive statistics were calculated for demographic and clinical characteristics at baseline; chi-square analyses for categorical variables and independent sample t-tests for continuous variables were used for comparison. Furthermore, patients were included in repeated univariate analyses of variance (ANOVA) model to examine the direction of changes (time effect) among groups (interaction effect) in terms of depressive symptom severity. This model was also used to analyze changes in hemodynamic measures, respiratory rate, oxygen saturation, and sedation from baseline to 120 min into surgery. All tests were two-sided and performed at a significance level of 0.05.
Results
Demographic and clinical characteristics of the sample at baseline. In total, 90 patients were recruited and randomized in the experimental group (n = 46) and the control group (n = 44). The mean age of the sample was 69.5 years (SD = 6.7, range 60-91), and 52.2% (n = 47) of the patients were male. Cataract was the most frequent diagnosis for the included patients (41.1%, n = 31). More than half of the patients had an ASA-PS functional capacity III before surgery, indicative of disabling disease. The demographic and clinical features by treatment group are shown in Table 1, which indicates that both groups were comparable at the time of their inclusion in the study.
Table 1: Demographic and clinical characteristics among groups at baseline assessment.
| Control (N = 44) | Ketamine (N = 46) | Statistics | p | |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Age (years)* | 69.4 ± 5.3 | 69.7 ± 7.9 | t = -0.21 | 0.83 |
| Midazolam dose (mg)* | 1.08 ± 0.5 | 0.90 ± 0.5 | t = 1.60 | 0.11 |
| Fentanyl dose (µg)* | 122.0 ± 43 | 106.5 ± 42.6 | t = 1.71 | 0.90 |
| Sex | χ2 = 2.8 | 0.09 | ||
| Male | 19 (43.2) | 28 (60.9) | ||
| Female | 25 (56.8) | 18 (39.1) | ||
| ASA functional capacity | χ2 = 0.61 | 0.73 | ||
| I | 1 (2.3) | 2 (4.3) | ||
| II | 17 (38.6) | 20 (43.5) | ||
| III | 26 (59.1) | 24 (52.2) | ||
| Diagnosis | χ2 = 7.6 | 0.10 | ||
| Cataract | 17 (38.6) | 20 (43.5) | ||
| Retinal detachment | 11 (25.0) | 3 (6.5) | ||
| Detachment + cataract | 6 (13.6) | 5 (10.9) | ||
| Vitreous hemorrhage | 2 (4.5) | 6 (13.0) | ||
| Hemorrhage + cataract | 8 (18.2) | 12 (26.1) | ||
| Type of surgery | χ2 = 3.4 | 0.48 | ||
| ECCE and IOL implantation | 12 (27.3) | 12 (26.1) | ||
| Vitrectomy | 13 (29.5) | 7 (15.2) | ||
| Vitrectomy + phacoemulsification | 4 (9.1) | 4 (8.7) | ||
| Vitrectomy + ECCE + IOL | 6 (13.6) | 10 (21.7) | ||
| Phacoemulsification | 9 (20.5) | 13 (28.3) |
ECCE = extracapsular cataract extraction; IOL = intraocular lens implantation;
SD = standard deviation.
* Values expressed as mean ± standard deviation; χ2 = Chi-square test; t = t-student test.
Ten patients were lost at the study’s final assessment stage (experimental group, n = 5; control group, n = 5) and were therefore excluded from ANOVA. Accordingly, five patients were excluded from the experimental group and five from the control group because they did not complete the third GDS-SF evaluation. Figure 1 shows the patient flow diagram and causes of exclusion. Completion rates between the experimental group (89.1%, n = 41) and the control group (88.6%, n = 39) were similar (χ2 = 0.006, p = 0.94).
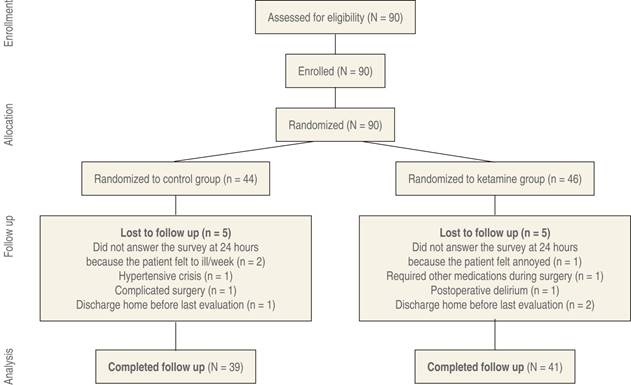
Figure 1: CONSORT flow chart indicating the selection, randomization and follow-up of the patients included in the study. Ten subjects were excluded due to intraoperative complications and personal concerns.
Depressive symptoms in elderly patients with visual disability: ANOVA model. Box’s test of equality of covariance matrices showed that the assumption of equal variances was not met (Box’s M = 22.8, p = 0.001), whereas the ANOVA model showed significant differences in the severity of depressive symptoms (according to GDS-SF) regarding time (Wilk’s lambda = 0.74, F = 13.0, p < 0.001) as well as a significant interaction effect between time and treatment group (Wilk’s lambda = 0.82, F = 8.3, p = 0.001). Figure 2 highlights the mean GDS-SF scores from baseline, postoperative, and 24-hour endpoint assessments for both treatment groups. As can be observed in Figure 2, both groups exhibited similar depressive symptom severity at baseline (ketamine, 4.3, SD = 2.8 vs. control, 3.5, SD = 3.3; t = -1.2, 88 df, p = 0.21); postoperatively, patients in the experimental group exhibited improvements in different symptoms when compared with the baseline assessment (mean change from baseline: ketamine, -0.91, SD = 1.6 vs control, -0.52, SD = 1.5; t = 1.1, p = 0.25). After 24 hours postoperatively, the difference reported in symptom improvement was greater in patients from the experimental group (mean change from baseline: -1.6, SD = 2.0) than in the control group (mean change from baseline: -0.3, SD = 1.4; t = 3.1, p = 0.003). For further confirmation of these results, we additionally performed ANOVA repeated-measures for each group, with Bonferroni correction, using the raw scores of GDS-SF from baseline to the 24-hour endpoint. No changes in the GDS-SF scores were observed regarding time in the control group (F = 1.83, 2 df, p = 0.17), whereas a significant reduction in the GDS-SF scores was observed in the experimental group (F = 21.0, 2 df, p < 0.001). These changes differed from baseline to postoperative assessment (p < 0.001) and then from the latter to the 24-hour endpoint (p < 0.001).
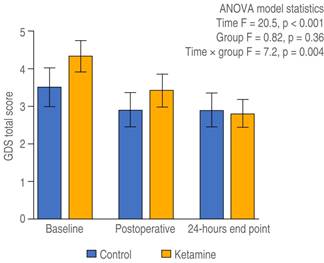
Figure 2: Depressive symptom severity in time among groups. The dispersion represents the standard error of the mean (SEM).
Hemodynamic measurements, respiratory rate, oxygen saturation, and sedation. For the present analyses, Box’s test of equality of covariance matrices showed that the assumption of equal variances was not met for any included variable (Box’s M p < 0.05). In contrast, the ANOVA model showed a significant effect in time, without differences between the placebo and the ketamine groups, and no significant interaction effects between time and treatment group (Table 2).
Table 2: Hemodynamic, respiratory rate, oxygen saturation and sedation measures.
| Placebo (N = 44) | Ketamine (N = 46) | Statistics | p | |
|---|---|---|---|---|
| Mean ± standard deviation | ||||
| Heart rate (bpm) | ||||
| Baseline | 69.6 ± 12.1 | 69.3 ± 14.0 | Time F = 12.4 | < 0.001 |
| 60 minutes into surgery | 64.8 ± 10.7 | 66.5 ± 11.7 | Group F = 0.3 | 0.570 |
| 90 minutes into surgery | 64.1 ± 14.5 | 66.2 ± 12.0 | Time × group F = 0.4 | 0.630 |
| Systolic blood pressure* | ||||
| Baseline | 159.2 ± 22.8 | 157.8 ± 23.5 | Time F = 31.5 | < 0.001 |
| 60 minutes into surgery | 145.4 ± 18.2 | 145.3 ± 18.7 | Group F = 0.3 | 0.550 |
| 90 minutes into surgery | 146.5 ± 17.0 | 141.9 ± 20.3 | Time × group F = 0.9 | 0.390 |
| Diastolic blood pressure* | ||||
| Baseline | 81.3 ± 10.0 | 82.1 ± 19.0 | Time F = 22.5 | < 0.001 |
| 60 minutes into surgery | 75.7 ± 10.6 | 74.8 ± 16.2 | Group F = 0.01 | 0.910 |
| 90 minutes into surgery | 75.9 ± 10.7 | 75.0 ± 15.4 | Time × group F = 0.3 | 0.690 |
| Mean arterial pressure* | ||||
| Baseline | 116.4 ± 14.3 | 115.9 ± 26.1 | Time F = 22.0 | < 0.001 |
| 60 minutes into surgery | 106.2 ± 12.8 | 104.7 ± 16.7 | Group F = 0.7 | 0.380 |
| 90 minutes into surgery | 106.0 ± 13.3 | 99.8 ± 22.0 | Time × group F = 1.0 | 0.350 |
| Oxygen saturation (%) | ||||
| Baseline | 93.1 ± 3.5 | 94.0 ± 4.0 | Time F = 11.4 | < 0.001 |
| 60 minutes into surgery | 98.1 ± 1.9 | 98.0 ± 2.1 | Group F = 1.5 | 0.210 |
| 90 minutes into surgery | 95.7 ± 15.2 | 98.0 ± 2.2 | Time × group F = 0.7 | 0.48 |
| Respiratory rate (bpm) | ||||
| Baseline | 15.9 ± 4.3 | 16.2 ± 3.7 | Time F = 50.0 | < 0.001 |
| 60 minutes into surgery | 11.9 ± 1.9 | 13.4 ± 2.3 | Group F = 3.7 | 0.060 |
| 90 minutes into surgery | 12.1 ± 2.8 | 13.1 ± 2.4 | Time × group F = 1.2 | 0.300 |
| Ramsay scale total score | ||||
| Baseline | 1.8 ± 0.3 | 1.8 ± 0.4 | Time F = 56.7 | < 0.001 |
| 60 minutes into surgery | 2.4 ± 0.5 | 2.3 ± 0.4 | Group F = 0.2 | 0.63 |
| 90 minutes into surgery | 2.4 ± 0.5 | 2.3 ± 0.5 | Time × group F = 0.3 | 0.69 |
* Data presented in mmHg.
Discussion
In the surgical context of elderly patients undergoing ophthalmological surgery, the use of ketamine for infusion at a dose of 0.5 mg/kg was associated with a significant reduction of depressive symptom severity, observed by a decrease in the GDS-SF scores. This effect was observed in the absence of reported adverse effects.
Although the use of ketamine has shown utility in reducing depressive symptoms, its use in elderly patients has been scarcely examined, with some studies showing a lack of efficacy. One study reported that IV ketamine administration had no significant effect on depressive symptoms but did have substantial adverse effects34. Further, these results have been explained by the effect of age on NMDA receptors. It has been shown that the process of aging is associated with a decrease in binding sites and the electrophysiological function of NMDA receptors35, especially in brain regions such as the frontal lobe and hippocampus, which are involved in learning and memory, functions affected by depression36. However, the results obtained in the present study are consistent with those obtained in at least one other study showing that ketamine can be useful for reducing depressive symptoms24.
Further, the effect of ketamine on depressive symptoms was observed in the absence of adverse effects. In up to 35% of studies, the use of ketamine has been found to be associated with cardiovascular, psychiatric, psychotomimetic, and neurological or cognitive effects37. In this study, hemodynamic reports showed no differences between the groups regarding hemodynamic variables during infusion and after the use of ketamine. This could be explained because of the small-dose ketamine given at an infusion rate of < 2.5 μg/kg-(1/min-(1, which is known does not cause hallucinations or impairment of cognitive functioning38. Additionally, the absence of adverse psychotropic and hemodynamic effects could be explained by the adjunctive use of midazolam, which has an anxiolytic and hemodynamic stabilizing effect39. Thus, the main psychotropic effects, such as anxiety, agitation, and dissociative symptoms, were not actively evaluated in this study. Therefore, it is not possible to eliminate neurological or cognitive symptoms as potential adverse events; hence, these factors should be assessed in future studies by adding other scales, such as the Hamilton Anxiety Rating Scale (HAM-A) and Clinician-Administered Dissociative Scale (CADSS). Midazolam has also been known to treat short-term anxiety and depression. The concomitant use could be a significant confounder; nevertheless, the study design helps us dispel this point since this medication was administered in small and similar dosages for both groups.
The strength of this study is that it addresses a hot topic and a common problem. In literature, there has been at least one randomized clinical trial evaluating the efficacy of ketamine treating depression symptoms in the elderly under major surgery and general anesthesia with negative outcomes40. Our results contrast, providing novelty evidence for at least a short-term effect of ketamine on the severity of depressive symptoms in a minor-sedative surgical context. Decreasing symptoms of depression after surgery can lead to a more noticeable improvement in less time; however, the latest statement must be explored in more depth in a future line of research.
The main limitations were that a standardized psychiatric diagnosis of major depressive disorder was not conducted, and the follow-up was not long enough to assess the duration of clinically meaningful effect. Although the GDS-SF scale has high sensitivity and specificity for detecting major depression41,42, it is always recommended to perform a face-to-face clinical interview with the patient to confirm the diagnosis because scales such as GDS-SF offer only screening for the presence of the disorder. Therefore, our results should be taken with caution because the observations only refer to the effect of ketamine on depressive symptoms severity and do not refer to the effect of ketamine on the phenotype of depression or resistant depression clearly. Despite the limitations mentioned above, the study contributes evidence about ketamine for reducing depressive symptoms in elderly patients in contexts not causally related to psychiatry. Examining evidence emerging from other non-psychiatric areas suggests the need for multidisciplinary work. It provides valuable information, particularly regarding the improvement of depressive symptoms that may have a negative impact on other medical conditions in elderly patients.
In conclusion, the administration of 0.5 mg/kg of ketamine as a single IV infusion was associated with improved depressive symptoms during the first 24 hours of exposure to the dose, with a good tolerability profile in elderly patients undergoing ophthalmological surgery. However, these results should be taken with caution until they are verified by further research.











 nova página do texto(beta)
nova página do texto(beta)


