Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista latinoamericana de química
versión impresa ISSN 0370-5943
Rev. latinoam. quím vol.41 no.2 Naucalpan de Juárez ago. 2013
Photodegradation of essential oil from marjoram (Origanum majorana L.) studied by GC-MS and UV-VIS spectroscopy
Jeovandro Maria Beltramea, Ricardo Almir Angnesa, Lucas Ulisses Rovigatti Chiavellib, Willian Ferreira da Costab, Maurício Ferreira da Rosac, Viviane da Silva Loboa, Armando Mateus Pominib,*
a Universidade Tecnológica Federal do Paraná. CEP 85902-040, Toledo, Paraná, Brasil.
b Universidade Estadual de Maringá, Departamento de Química. CEP 87020-900, Maringá, Paraná, Brasil. *Corresponding author. ampomini@uem.br; armandopomini@gmail.com. Phone: + 55 44 3011 3664; Fax + 55 44 3011 4125.
c Universidade Estadual do Oeste do Paraná, Curso de Química. CEP 85903-000, Toledo, Paraná, Brasil.
Received January 2013.
Accepted July 2013.
ABSTRACT
Marjoram (Origanum majorana L.) is an aromatic plant originating from European and African continents whose essential oil possesses anti-inflammatory and inhibitory activity against bacteria, besides the large use as food additive. However, there is a lack of information regarding to the photochemical stability of the essential oil and its degradability. Thus, this study aimed to obtain information about the photodegradation profile of marjoram essential oil, since this is a very important factor for the storage and preservation of the product. As results, 2-undecanone and p-diisopropyl-benzene were identified as the main products of essential oil photodegradation. It may be an important information for future quality control protocols, since these substances are not found in fresh essential oil. www.relaquim.com
Keywords: marjoram, Origanum majorana, photodegradation, 2-undecanone.
RESUMEN
Mejorana (Origanum majorana L.) es una planta aromática procedente de los continentes europeos y africanos cuyo aceite esencial posee actividad anti-inflamatoria, anestésico y inhibitoria contra bacterias, además de la utilización a gran como aditivo alimentario. Sin embargo, hay una falta de información con respecto a la estabilidad fotoquímica de los aceites esenciales y su degradabilidad. Por lo tanto, este estudio tuvo como objetivo obtener información sobre el perfil de la fotodegradación de aceite esencial de mejorana, ya que este es un factor muy importante para el almacenamiento y la conservación del producto. Como resultados, 2-un-decanona y diisopropil-benceno son los principales productos de fotodegradación de lo aceite esencial. Puede ser información importante para futuros protocolos de control de calidad, ya que estas sustancias no se encuentran en el aceite esencial fresco. www.relaquim.com
Palabras clave: mejorana, Origanum majorana, fotodegradación, 2-undecanona.
INTRODUCTION
Marjoram (Origanum majorana L., Lamiaceae) is a perennial plant native of southern Europe but cultivated worldwide. This plant has widespread use in cooking and is found in many dishes as a condiment. The commercial value of this plant is significant in Brazil, mainly for small farmers (Embrapa, 2007).
This plant is cultivated or collected from natural fields in the Mediterranean region since ancient times. A broad analysis performed with marjoram native from Cyprus revealed the presence of trans-sabinene hydrate/terpinen-4-ol or α-terpineol/ trans-sabinene hydrate as the main chemical components found in essential oil produced from this species (Karosou et al., 2012). An ecophysiological study showed that O. ma-jorana essential oil content varies according to harvest at different phenological stages. Results showed that later vegetative stage is characterized by the highest contents of essential oil and therefore it could be considered as the best stage for harvesting marjoram plants (Sellami et al., 2009). Moreover, in silico cultivations with salt constraints can significantly change the essential oil yield and composition (Baâtour et al., 2012). Finally, the yields of essential oil from O. majorana changes considerably according to the production technique employed, being supercritical extraction with carbon dioxide the most effective procedure reported (Vági et al., 2005).
In a previous study, it was observed that O. majorana Brazilian cultivars have terpinen-4-ol, y-terpinene, p-cimene and a-terpinene as the main essential oil components, which in turn showed an interesting antibacterial activity against E. coli, moderate activity against S. enterica and E. sakazakii, and was inactive against L. monocytogenes (Valeriano et al., 2012).
The photosensitivity is the alteration of chemical substances structures under exposition to light. One of the factors that can alter the chemical composition of an essential oil is photodegradation. Once obtained from natural sources, essential oils can undergo this process under storage, transportation or use, compromising their organoleptic properties, toxicity patterns or its applicability as antimicrobial agents. On the other side, the photodegradation procedures may originate new chemical compounds with interesting properties and uses.
The present work aimed to evaluate the chemical composition, photodegradation pattern and antimicrobial activity of essential oil of marjoram grown in Brazil, considering the importance and current consumption of its essences (either directly or indirectly) in foods, condiments and as a income for small farmers in countryside.
MATERIALS AND METHODS
EQUIPMENT
The commercial dry plant material was subjected to extraction by steam distiller in stainless steel Clevenger SL 76 Solab Scientific apparatus. It was circumstantially dried in an oven with steam circulation and air exchange Solab SL 102/125. Essential oils were analyzed by GC-MS using a Thermo-Finnigam equipment, model Focus DSQ II, fitted with Agilent DB-5 capillary column (30 m x 0.25 mm x 0.25 jam). The temperature of the ionization source was of 220 °C, injector at 220 °C, split injection mode 1/50. The carrier gas used was highly pure helium at 1.0 mL / min. It was injected 1 µL of sample dissolved in dichloromethane. The equipment operated in EI (70 eV) mode with mass spectral range 50-650 u. The retention indices (RI) comparative to hydrocarbons were determined using the equation of van der Dool and Kratz according to procedure described in the literature (Collins, 2006).
PLANT MATERIAL AND ESSENTIAL OIL
EXTRACTION
Samples of dried marjoram (Origanum majorana L.) were obtained by direct purchasing in commerce site, aiming to obtain a sample that was both homogenous and representative of the chemical profile of the plant commonly found and marketed in Brazil.
The essential oil was obtained using Clevenger distillation apparatus. The process employed 5 L of distilled water for 500 grams of plant dry mass. The essential oil yield was calculated from the weight of essential oil obtained, based on the initial mass of the plant, and also fixed to the yield on dry vegetable mass.
For correct yield determination, it was necessary to determine the moisture of the dry material obtained commercially. Thus, a moisture scale determiner with infrared drying BEL was employed. The drying procedure was adjusted at 105 oC using the automatic stop tool, when minimum deviation of humidity (± 0.1%) was not reached during 30 seconds.
PHOTODEGRADATION ASSAYS
Tests of essential oil photodegradation were carried out using a photoreactor equipped with a mercury lamp (125 W). The outer bulb was removed to permit the emission of ultraviolet light. These tests were employed for spectroscopic studies of essential oil degradation. In general, solutions containing the essential oil were exposed to the light from the lamp previously described, at a distance of 20 cm for five minutes, with spectrophotometer readings taken every minute. Readings were performed using quartz cuvette with optical path of 10 mm and a UV-VIS GENESIS UV model 10 spec-trophotometer, scanning in the range of 190-350 nm. The degradation pattern was tested in three solvents (dichloromethane, ethanol and hexane) to oils derived from plants in concentrations of 80 µL/mL. The spectra were deducted from solvents degradation pattern. For accelerated photodegradation studies a more potent lamp (250 W) without bulb was employed. The essential oil solutions (30 µL/mL) in dichloromethane and hexane were exposed for 5 minutes to UV light in quartz cuvettes in the same conditions described above and samples analyzed by GC-MS.
ESSENTIAL OIL ANALYSES
Separation and identification of chemical constituents of the essential oil were performed by gas chromatography-mass spectrometry using the gradient column temperature: 60 °C (2 min), 5 ° C/min to 110 oC; 3 °C/min to 150 °C, 3 °C/min to 15 °C/min to 280 °C (6 minutes). The samples were standardized to 0.001 g/L using dichloromethane as solvent and with the injection of 1 µL of solution.
The identification of essential oil components was carried out by mass spectral data comparison with NIST library MS Search 2.0. In addition, the retention indexes related to hydrocarbons standard were determined and the results compared with literature (Adams, 2007).
ANTIBACTERIAL ACTIVITY EVALUATION
Minimal inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) tests for marjoram essential oils were performed against Staphylococcus aureus (ATCC-25923), Bacillus subtilis (ATCC-6623), Escherichia coli (ATCC-25922) and Pseudomonas aeruginosa (ATCC-27853), using routine MIC procedures. Each microorganism solution was standardized in comparison with a solution of 0.5 McFarland scale and diluted ten-fold for inoculum preparation. The concentrations of the essential oil of 1000, 500, 250, 125, 62.5, 32.25 and 15.625 mg/ml were tested, using Mueller-Hinton cultivation media. The biological activities were evaluated after incubation for 24 hours under controlled temperature.
RESULTS AND DISCUSSION
The extraction of essential oil of marjoram was performed in triplicate and the average yield corrected for dry plant mass was 0.47 ± 0.14 %. The moisture of the commercial dried plant was by 12.96 %. The yield of essential oil of marjoram was slightly below the value found in the literature (1.1 %) possibly due to storage for long periods of time at local commerce (Rodrigues, 2002).
After obtaining the oil, analyzes were conducted to determine its chemical composition by gas chromatography coupled to mass spectrometry and by comparing the retention indexes of the substances with literature. Results are shown in Table 1. Derivatives of terpinene and terpineol were the most abundant substances in the essential oil obtained from the samples employed in this study, in agreement with revised literature (Valeriano et al., 2012; Karosou et al., 2012).
Tests of photodegradation of the essential oil of marjoram were performed to evaluate their stability under exposure to ultraviolet-visible light. The photodegratation spectra are shown in Figure 1. Moreover, the degradation profile can be attested by great changes in chromatographic pattern of fresh essential oil and photodegradated samples, as can be attested in Figure 2.
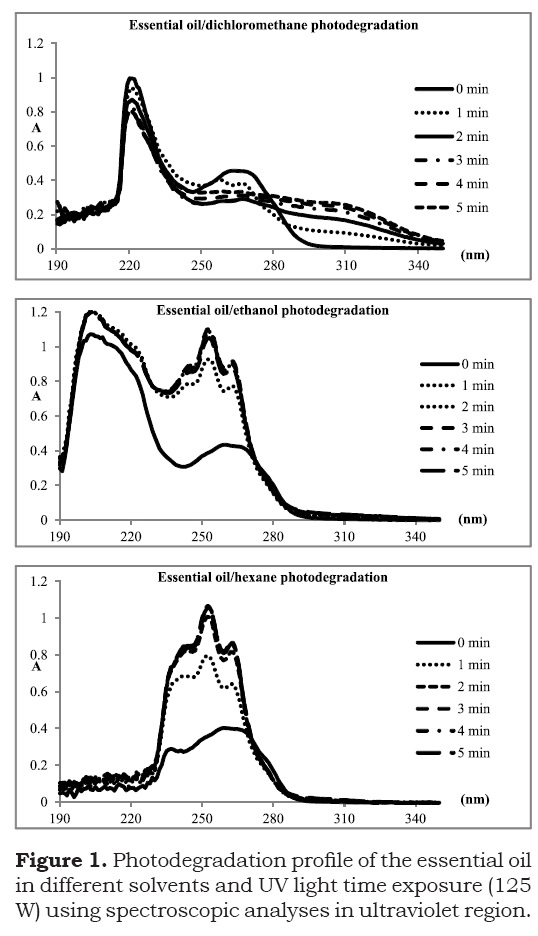
According to the UV-VIS spectra, it was found that dilution of the essential oil in hexane and ethanol to a large extent facilitates the photodegradation process with increase in absorbance over time in the region between 240-280 nm. On the other side, the exposition of dichloromethane/ essential oil solution to light radiation provided a minor change in the levels of absorbance of the sample. It is therefore clear that the solvent medium alters the pattern of the essential oil decomposition by ultraviolet radiation.
A bathocromic shift was observed when exposure to ultraviolet light was performed with the essential oil dissolved in dichloromethane. An increase in absorbance intensity in the region between 290-330 nm attests the effect. Moreover, the experiments with the essential oil dissolved in ethanol and hexane resulted in a clear hyperchromic effect for the band at 230-280 nm, while no extensive bathocromic shifts were observed. Interestingly, the absorbance profile for the photodegradation in ethanol and hexane were very similar, an indication that the same chemical degradation mechanisms may be observed in both non-polar hexane and polar methanol solvent.
The result with ethanol is particularly interesting, since the use of this solvent in the preparation of dye solutions of essential oils is common. Thus, it is evident the need for preservation of samples or batches of commercial essential oils in the dark, given the rapid degradation even in the presence of ultraviolet radiation. Furthermore, the use of amber or opaque vials can further contribute to the preservation of the same order transport and storage, especially with commercial focus.
Aiming to better understand the origin of these effects, analyzes of photodegradation products were performed by gas chromatography-mass spectrometry with solvents hexane and dichloromethane. In both cases, the monoterpenes (C10) present in the fresh essential oil originated mainly 2-undecanone (C11) and p-diisopropyl benzene (C12) as degradation products (Table 2, Figure 3). Differences in carbon numbers and molecular structures from the reactants to products clearly show that complex intermolecular reactions may took place under UV catalysis.
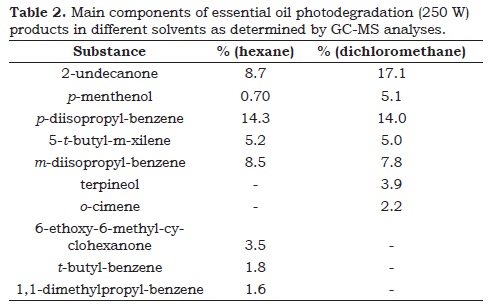
Based on these results, it is clear that the dichloromethane has a slightly greater ability to protect the natural monoterpenes of photodegradation under UV light, due to the reminiscence of these products after the degradation process. In the case of hexane, it was practically not observed remnants of the original monoterpenes after photodegradation at the same conditions. Furthermore, the photodegradation in hexane produced a higher concentration of aromatic products than ketones, thus explaining the hyperchromic effect observed during the reaction. Since the reaction in dichloromethane was able to favor the formation of 2-undecanone to a greater extent than the aromatic derivatives, it explains the more intense bathocromic shift observed in this solvent.
These experiments provide important information concerning to production of marjoram essential oil, given the extensive chemical alteration observed under accelerated light exposure. It reinforces the importance of good manufacturing practices, storage and transport of both plant material and the oil obtained, since their properties can be seriously compromised in this process. Once known the main photodegradation products, future control quality protocols for marjoram essential oil can be established. As essential oils have emerged as an alternative employment and income for small farms, these findings assume an important role regarding the economic development.
More interestingly, the main photodegradation product 2-undecanone is a bioactive substance against the potato and tomato plague Phthorimaea operculella, considerably less toxic than commercial agricultural defensives (Ventura and Vendramin, 1995). It may open an opportunity for production of green and more active substances for agricultural production against plant consuming caterpillars from essential oils using photodegradation procedures.
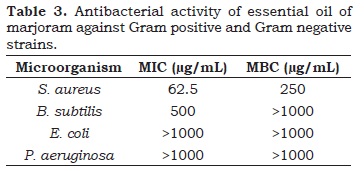
Finally, it was carried out the evaluation of the antimicrobial activity of essential oil against important bacterial strains. Results are shown in Table 3. The essential oil of marjoram had inhibitory activity to the microorganism B. subtilis at a concentration of 500 mg / mL. Moreover, it displayed inhibitory and bactericidal activities at the respective concentrations of 62.5 mg/ mL and 250 mg/mL for S. aureus (Table 3). The positive antimicrobial activity is in agreement with results observed for other Origanum species (Esen et al., 2007).
CONCLUSION
This work reports for the first time the photodegradation chemical profile of marjoram essential oil, showing that extensive and complex intermolecular reactions catalyzed by UV light generated mainly 2-undecanone and p-diisopropyl-benzene isomers as products. It may be a important information for future essential oil quality control protocols development. Moreover, the antimicrobial activity of the essential oil against important bacteria was also evaluated, reinforcing the importance of these substances for future uses as antimicrobials.
REFERENCES
Adams, R. P. (2007) Identification of essential oil components by gas chromatography/mass spectrometry. 4th Edition. Allured Publishing Corporation, Carol Stream, IL, USA. [ Links ]
Baâtour, O., Kaddour, R., Tarchoun, I., Nasri, N., Mahmoudi, H., Zaghdoudi, M., Ghaith, H., Marzouk, B., Nasri-Ayachi, M. B., Lachaál, M. (2012) Modification of fatty acid, essential oil and phenolic contents of salt-treated sweet marjoram (Origanum majorana L.) according to developmental stage. Journal of Food Science, 77: 1047-1050. [ Links ]
Collins, C. H. (2006) Fundamentos de cromatografía. Editora Unicamp. Campinas, São Paulo, Brazil, pp. 456. [ Links ]
Embrapa. (2007) Manjerona - Série Plantas Medicinais, Condimentares e Aromáticas. Corumbá, MS, Brazil. [ Links ]
Gülden, E., Azaz, A. D., Kurkcuoglu, M., Baser, K. H. C., Tinmaz, A. (2007) Essential oil and antimicrobial activity of wild and cultivated Origanum vulgare L. subsp. hirtum (Link) letswaart from the Marmara region, Turkey. Flavour and Fragrance Journal, 22: 371-376. [ Links ]
Karousou, R. Efstathiou, C., Lazari, D. (2012) Chemical diversity of wild growing Origanum majorana in Cyprus. Chemistry & Biodiversity, 9: 2210-2217. [ Links ]
Rodrigues, M. R. A. (2002) Estudo dos óleos essenciais presentes em manjerona e orégano. Ph.D. Thesis, UFRGS, Porto Alegre, RS, Brazil. [ Links ]
Sellami, I. H., Maamouri, E., Chahed, T., Wannes, W. A., Kchouk, M. E., Marzouk, B. (2009) Effect of growth stage on the content and composition of the essential oil and phenolic fraction of sweet marjoram (Origanum majorana L.). Industrial Crops and Products, 30: 395-402. [ Links ]
Vági, E., Simándi, B., Suhajda, Á., Héthelyi, É. (2005) Essential oil composition and antimicrobial activity of Origanum majorana L. extracts obtained with ethyl alcohol and supercritical carbon dioxide. Food Research International, 38: 51-57. [ Links ]
Valeriano, C., Piccoli, R. H., Cardoso, M. G., Alves, E. (2012) Antimicrobial activity of essential oils against sessile and planktonic pathogens of food source. Revista Brasileira de Plantas Medicinais, 14 : 57-67. [ Links ]
Ventura, M. U., Vendramim, J. D. (1995) Toxicidade para lagartas de Phthorimaea operculella (Zell.) dos aleloquímicos 2-tridecanona e 2-undecanona presentes em tomateiro (Lycoper-sicon spp.). Scientia Agrícola, 52: 458-461. [ Links ]














