Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista latinoamericana de química
versión impresa ISSN 0370-5943
Rev. latinoam. quím vol.40 no.3 Naucalpan de Juárez dic. 2012
Synthesis and chemical-optical characterization of push-pull stilbenes
Blanca M. Muñoz-Floresa, Rosa Santillánb, Mario Rodríguezc, Gabriel Ramosc, José Luis Maldonadoc, Margarita Romerod, Norberto Farfánd,*
a Facultad de Ciencias Químicas, Universidad Autónoma de Nuevo León, Av. Pedro de Alba s/n, 66451 San Nicolás de los Garza, N. L., México.
b Departamento de Química, Centro de Investigación y de Estudios Avanzados del IPN, 07000, Apdo. Postal. 14-740, México D. F., México.
c Centro de Investigaciones en Óptica A.P. 1-948, 37000 León, Gto., México.
d Facultad de Química, Depto. de Química Orgánica, Universidad Nacional Autónoma de México, 04510, México, D.F., México.
Received October 2012.
Accepted December 2012.
ABSTRACT
In this work we report the synthesis and spectroscopic characterization of push pull stilbenes. These compounds were prepared in good yield and characterized by 1H and 13C NMR, IR and MS. Single-crystal X-ray diffraction analysis of acids 1 and 2 evidenced the existence of intermolecular hydrogen bonding which in the case of compound 1 [(Z)-isomer] involves interaction between the acid group and a methoxy group other neighbor molecule in contrast for in (E)-isomer (2) the intermolecular hydrogen bonding is between the acid groups promoting the formation of centrosymmetric dimmers. From acids 1 and 2 was obtained the ester 3, for which was improved its solubility properties. Lineal and non linear optical characterization of ester derivative was carried out in particular the second molecular hyperpolarizability using the Maker-Fringe technique.
Keywords: Stilbene, push-pull, nonlinear optics, NLO, X-ray diffraction.
RESUMEN
En este trabajo se describe la síntesis y la caracterización espectroscópica de nuevos compuestos estilbenos tipo donador-Π-aceptor (push-pull). Dichos compuestos fueron obtenidos en buenos rendimientos y caracterizados por RMN de hidrógeno y carbono, la espectrometría de masa muestra el ion molecular y para el caso de los ácidos marcados como 1 y 2, fue posible establecer su estructura mediante difracción de rayos-X. En ambos compuestos se encuentran presentes interacciones por puente de hidrógeno, para el caso del compuesto 1 (isómero Z) dicha interacción se da entre el grupo ácido y el grupo metoxilo, mientras que en el compuesto 2 (isómero E) el puente de hidrógeno intermolecular es entre los grupos ácidos. Finalmente para el éster 3, fue posible medir la segunda hiperpolarizabilidad molecular mediante la técnica Maker-Fringe.
Palabras clave: Estilbenos, donador-π-aceptor (push-pull), óptica no lineal, ONL, difracción de rayos-X.
INTRODUCTION
Nonlinear optical (NLO) properties were first observed in the decade of the sixties after the discovery of the laser by Franken et al. (1961), and certainly inorganic compounds have been the most studied and used in the manufacture of optoelectronic devices. However, in the last two decades organic nonlinear optical materials have been greatly investigated by Goto et al. (1991) due to their potentially high nonlinearities and rapid response in electro-optic effect compared to inorganic NLO materials. Moreover, these compounds are structurally modifiable and allow modulation of the NLO effect, in contrast to inorganic compounds where this is not possible. Also, organic compounds show other advantages over inorganic ones such as low-refractive index, their polarizabilities are purely and in particular they have lower processing cost.
It is well known that organic compounds that present NLO responses must contain into their molecular structure an electron donor and an acceptor group bonded by a conjugated n electron system. For this reason, the design of push-pull chromophores has been based on a dipolar D-p-A structural motif (Figure 1).

In order to understand the microscopic origin of the nonlinear behavior of organic NLO materials, considerable theoretical and experimental investigations have been developed by Kerkoc et al. (1990) and Dimitriev et al. (1991). The conjugated Π electron system provides a pathway for the entire length of conjugation under the perturbation of an external electric field. Functionalization of both extremes of the p conjugated system with electron donor and acceptor groups could increase the asymmetric electronic distribution in the ground and excited states, thus leading to an increased optical non-linearity (Prasad et al. 1991). In continuation with our studies, we report herein the synthesis, characterization and NLO properties of (E)- and (Z)-3-(4-methoxyphenyl)-2-(4-nitrophenyl)-acrylic acids and the corresponding (E)-ethyl ester (3) (Scheme 1). All compounds were fully characterized by IR, UV, NMR, MS and in the case of derivatives 1 and 2 their structures were corroborated by X-ray diffraction analysis.

Compounds 1 and 2 were obtained by reaction of p-anisaldehyde and p-nitrophenylacetic in acetic anhydride and triethylamine. Both compounds were found to be poorly soluble in most organic solvents, for this reason it was difficult to study their NLO properties. In order to improve their solubility to measure NLO properties, the corresponding ethyl ester derivatives were synthesized by reaction of the acid with thionyl chloride and ethanol. It should be mentioned that when compound 1 was treated under the same reaction conditions, the product undergoes isomerization to give compound 3 (Scheme 1).
MATERIALS AND METHODS
Instruments
All starting materials were commercially available. Solvents were used without further purification. Melting points were recorded on an Electrothermal 9200 apparatus and are uncorrected. Infrared spectra were measured on a FT-IR Perkin-Elmer GX and Perkin Elmer 400 spectrophotometer using KBr pellets and ATR. 1H, and 13C NMR spectra were recorded on Jeol Eclipse +400 and Varian Unity 300 spectrometers. Chemical shifts (ppm) are relative to (CH3)4Si for 1H and 13C. Ultraviolet spectra were obtained with a Perkin-Elmer Lambda 2 spectrophotometer. Mass spectra were recorded on a Hewlett-Packard 5989A spectrometer and FAB Thermo-Electron Model: DFS (Double Focus Sector). Elemental analyses were carried out on a Thermo Finnigan Flash EA 1112 elemental microanalyzer.
X-Ray data collection and structure determination
For acids 1 and 2 single crystals suitable for X-ray structural studies were obtained by slow evaporation from mixtures of CHCl3 and hexane. The crystal data were recorded on an Enraf Nonius Kappa-CCD (Mo Ka=0.71073 Å, graphite monochromator, T=293 K CCD rotating images scan mode). The crystals were mounted on a Lindeman tube. Absorption corrections were performed using the SHELX-A (Sheldrick et al. 1997) program. All reflection data set were corrected for Lorentz and polarization effects. The first structure solution was obtained using the SHELXS-97 program and then SHELXL-97 program was applied for refinement and output data (Sheldrick et al. 1997). All software manipulations were done under the WIN-GX environment program set (Farrugia et al. 1999). Molecular perspectives were drawn under ORTEP3 drawing application. (Farrugia et al. 1997) All heavier atoms were found by Fourier map difference and refined anisotropically. Some hydrogen atoms were found by Fourier map differences and refined isotropically. The remaining hydrogen atoms were geometrically modeled and are not refined.
Syntheses
A solution of 5.44 g (0.040 mmol) of p-anisaldehyde and 6.69 g (0.037 mmol) of p-nitrophenylacetic acid in 4 ml of triethylamine and 4 ml of acetic anhydride was heated under reflux in an oil bath maintained at 150 °C for 4 h. A precipitate often forms during this heating period. The reaction mixture was acidified with 8 ml of concentrated hydrochloric acid, and the organic phase was extracted with 300 ml of methylene chloride and water. This solution was washed with several 100 ml portions of water and then extracted with three 100 ml portions of 20% sodium hydroxide. The combined extracts were then acidified to pH of 4.0 with about 15 ml of acetic acid. The trans acid was filtered from the solution containing the salt of the cis acid and washed with water. The cis isomer was precipitated by the addition of 15 ml of concentrated hydrochloric acid.
(Z)-3-(4-methoxyphenyl)-2-(4-nitrophenyl)-acrylic acid (1) (Kertcham et al. 1963)
The product was obtained as an orange solid (2.25 g 7.52 mmol), yield 20%, m.p. 234-236 °C. IR (KBr) vmax (cm-1): 3448 (OH), 2937, 2841, 2512, 2040, 1669 (CO), 1596, 1511, 1426, 1346, 1257, 1172, 1106, 1025, 923, 854, 831, 784, 711, 654, 551; MS m/z (%), 300 (M++1, 19), 299 (M+, 100), 283 (2), 269 (3), 254 (6), 208 (13), 165 (39), 137 (54), 109 (14); 1H-NMR (400 MHz, DMSO--d6), δ (ppm): 3.70 (3H, s, OCH3), 6.79 (2H, d, J=9.0 Hz, H-3, H-5), 6.99 (2H, d, J=9.0 Hz, H-2, H-6), 7.47 (2H, d, J=8.8 Hz, H-11, H-15), 7.81 (1H, s, H-7), 8.24 (2H, d, J=8.8 Hz, H-12, H-14); 13C-NMR (100 MHz, DMSO-d6), δ (ppm): 55.8 (OMe), 114.7 (C-3, C-5), 124.3 (C-12, C-14), 126.6 (C-1), 129.4 (C-8), 131.8 (C-11, C-15), 132.8 (C-2, C-6), 140.8 (C-7), 144.9 (C-10), 147.3 (C-13), 160.8 (C-4), 168.2 (C-9); Elemental analysis calcd. for C16H13NO5: C 64.21, H 4.38, N 4.68. Found: C 64.34, H 4.13, N 4.60.
(E)-3-(4-methoxyphenyl)-2-(4-nitrophenyl)-acrylic acid (2) (Kertcham et al. 1963)
The product was obtained as a yellow solid (0.84 g 2.80 mmol), yield 8%, m.p. 193-196 °C. IR (KBr) vmax (cm-1): 3445 (OH), 2339, 1722 (CO), 1589, 1513, 1397, 1340, 1248, 1178, 1111, 1011, 850, 823, 753, 570; MS m/z (%), 300 (M++1, 18), 299 (M+, 100), 283 (3), 269 (10), 254 (4), 208 (7), 165 (8), 137 (25), 109 (5); 1H-NMR (300 MHz, DMSO-d6), δ (ppm): 3.80 (3H, s, OCH3), 7.01 (2H, d, J=8.8 Hz, H-3, H-5), 7.30 (1H, s, H-7), 7.53 (2H, d, J=8.8 Hz, H-2, H-6), 7.76 (2H, d, J=8.9 Hz, H-11, H-15), 8.27 (2H, d, J=8.9 Hz, H-12, H-14); 13C-NMR (68 MHz, DMSO-d6), δ (ppm): 55.8 (OMe), 114.7 (C-3, C-5), 124.6 (C-12, C-14), 127.2 (C-11, C-15), 127.8 (C-1), 130.9 (C-2, C-6), 131.8 (C-7), 132.8 (c-8), 143.7 (C-10), 146.9 (C-13), 160.5 (C-4), 170.8 (C-9); Elemental analysis calcd. for C16H13NO5: C 64.21, H 4.38, N 4.68. Found: C 64.34, H 4.13, N 4.60.
Ethyl (E)-3-(4-methoxyphenyl)-2-(4-nitrophenyl)-acrylate (3)
The reaction flask was first charged with a mixture of compounds 1 and 2 (450 g, 1.5mmol) in 20 ml thionyl chloride and refluxed for 2 h under N2 atmosphere, excess thionyl chloride was eliminated by distillation followed by addition of 20 ml of ethanol and the solution was stirred for 24 hr. The solvent was evaporated, and ethyl acetate was added. After drying over anhydrous Na2SO4 and purification by column chromatography with a hexane:ethyl acetate mixture (9:1), the product was obtained as a yellow solid 0.45 g (91% yield) mp 77-79 °C. IR (ATR) vmax (cm-1):2987, 2940, 1682 (CO), 1597, 1509, 1343, 1343,1245,1172,1029,853, 835 MS m/z (%), 344 (M++1, 42), 327 (M+, 82), 282 (50), 254 (40), 254 (4), 208 (20), 165 (44), 136 (30), 73 (100); 1H-NMR (400 MHz, Cl3CD), δ (ppm): 1.29 (3H, t, J=7 Hz, CH3), 3.76 (3H, s, OCH3), 4.26 (2H, q, J=7.0 Hz, CH2), 6.97 (2H, d, J=8.9 Hz, H-3, H-5), 7.42 (2H, d, J=8.8 Hz, H-2, H-6), 7.42 (2H, d, J=8.9 Hz, H-11, H-15), 7.90 (1H, s, H-7), 8.24 (2H, d, J=8.8 Hz, H-12, H-14); 13C-NMR (100 MHz, Cl3CD), δ ppm: 14.2 (CH3),55.3 (OMe), 61.3 (CH2) 114.0 (C-3, C-5), 123.8 (C-12, C-14), 126.2 (C-1), 128.2 (C-8), 131.2 (C-11, C-15), 132.4 (C-2, C-6), 141.6 (C-7), 143.7 (C-10), 147.2 (C-13), 160.7 (C-4), 166.7 (C-9);
Third non linear optical measurements for compound 3
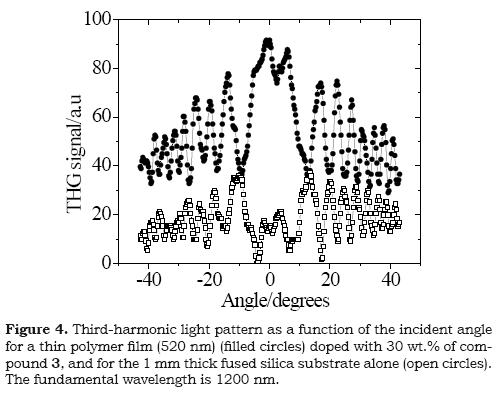
The third nonlinear response susceptibility for compound 3 was measured as macroscopic nonlinear polarizability (x(3)) by the Maker-Fringe technique over polymeric film doped. The sample was prepared using the guest(molecule)-host(inert polymer) approach using polystyrene. Ratios of 70:30 wt % of polystyrene and chromophore 3 were dissolved in chloroform. The solid polymeric films were deposited over fused silica substrates (1 mm-thick) by using the spin coating technique. The films of 3 had a thickness of 520 nm, with a good optical quality at visible and NIR wavelength. The thickness of the polymeric film was measured using a Dektak 6M profiler. The THG Maker-Fringes technique consists on the comparison of the oscillations in the THG intensity produced by the substrate alone and those obtained from the active film on a substrate, as a consequence of the variations in the incident angle of the pumping laser beam. Details of this experiments and THG Maker-Fringes setup can be found in the literature (Muñoz et al. 2008).
RESULTS AND DISCUSSION
Spectroscopic data
All compounds were characterized by 1H and 13C NMR experiments, selected data are summarized in Table 1. The singlets at 7.30, 7.81 and 7.90 ppm in the 1H-NMR spectra of compounds 1 , 2 and 3 correspond to the vinylic protons.

The 1H-NMR signals for the aryl rings were assigned based on their COSY spectra which allowed correlation between the protons in the ring containing the electron donor group and those of the ring with the electron acceptor group which gave two AX systems with coupling constants between 8.8 and 8.9 Hz. In the 13C-NMR spectra of compounds 1 , 2 and 3, the signals for C-4 and C-9 are shifted to high frequencies (160.5, 160.8 and 160.7 ppm for C-4 and 170.8, 168.2 and 166.7 ppm for C-9). The two isomeric acids could be identified based on the characteristic chemical shift observed for the vinylic carbon (C-7) which in (E)-isomer shows a marked deshielding effect (140.8 ppm) compared to (Z)-isomer (131.8 ppm). The same trend is observed for H-7 in the proton spectra. In the IR spectra, the carbonyl band appears at 1668 and 1669 cm-1for the (E)-isomers and 1722 cm-1 in the (Z)-isomer.
Molecular structure
The acids 1 and 2 were crystallized by slow evaporation of a concentrated mixture of chloroform and hexane and the molecular perspectives are shown in Figure 2.
The details of the crystal data and summary of the collection parameters for acids 1 and 2 are given in Table 2. Selected bond distances and angles are compared in Table 3


One important feature that the two compounds share is the presence of intermolecular hydrogen bonding, in the case of compound 1 [(Z)-isomer], this interaction is between the carboxyl group and the methoxy group of a neighboring molecule with bond distance of 1.917 (2) Å. In contrast, in compound 2 [(E)-isomer] this intermolecular hydrogen bonding is between the acid groups of neighbor molecules with a bond distance of 1.384 (2) Å forming a dimeric centrosymmetric structures (Figure 3).
Several differences are identified in the relative configurations of the two compounds; Table 3 shows selected dihedral angles and torsion angles for specific fragments of each molecule. From these data it is evident that (E)-isomer (2) is considerably more sterically crowded, and as a consequence the two rings twist out of plane formed by H7-C7-C8 atoms, showing a dihedral angle of 55.7° between the planes of the two rings, as well as a torsion angle of 4.3° for the C6-C1-C7-H7 fragment.
Lineal absorption of compound 3
The lineal optical characterization of compound 3 was carried out in solution using solvents with different polarities. The absorption spectrum showed a broad absorption band with a maximum peak around 291 nm. A short blue shift (6 nm) is shown for the absorption band of 3 from non polar (toluene) to polar solvents (methanol). The absorption band is due to n→Π* electronic transition over the Π-backbone system, which is in agreement with the push-pull architecture present on compound 3. The position of the electronic transition band in the absorption spectra is affected directly for the cis configuration of the double bond, reducing the conjugation process over the n-system (Nalwa et al.1997).
Third non linear optical properties
The cubic non-linear (macroscopic) response of compound 3 was measured over solid film using the third harmonic generation (THG) Maker-Fringes technique at the IR wavelength (1200 nm), and the typical graph for THG responses obtained in this technique is shown in Figure 4. For derivatives 1 and 2 their insolubility properties in common organic solvents precluded optical characterization in solid film. The macroscopic nonlinear susceptibility x (3) was used to calculate the second hyperpolarizability γ, which is the molecular parameter of interest, through γ = x (3)/L4Ns where Ns is the density of molecules in the polymer film. L= (n2+2)/3 is the correction factor due to local field effects and n is the refractive index. Assuming for the film the refractive index and density of polystyrene, and using a molecular doping level of 30%, the y value for compound 3 resulted to be 763 x 10-36 esu, which is in the is longer than the values calculated for other derivatives of stilbene (63-228 x 10-36 esu) (Romaniello et al. 2004). It has been reported that the non linear responses of organic molecules are related to the polarization of the π-electronic system which is dependent on structural parameters such as the length of the π-backbone and configuration (Bredes et al. 1994). The non linear response measured for derivative 3 is affected directly by the E configuration of the stilbene, which could be responsible for the low value.
CONCLUSIONS
The isomeric push-pull acids (Z)-1 and (E)-2 were prepared and characterized. Attempts to prepare the ethyl ester derivatives in order to increase their solubility and to study their. NLO properties lead to isomerization of (Z)-1 to the (E)-isomer (compound 3). The non linear response measured for derivative 3 is affected by the Z configuration of the aromatic rings in the stilbene, which could be responsible for the low value observed. However, experimental second hyperpolariability obtained for compound 3 is longer than those values theoretically estimated for other stilbene derivatives.
CCDC-905466 and CCDC-905467 contain the supplementary crystallographic data for 1 and 2, respectively. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre 12, Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223 336033; or e.mail: deposit@ ccdc.cam.ac.uk.
ACKNOWLEDGEMENTS
The authors thank UNAM (PAPIIT IN-214010) and CONACyT for financial support. The author thanks Rafael Espinosa for the film thickness measurements.
REFERENCES
Brédas, J.L., Adant, C., Tackx, P., Persoons, A. (1994) Third-Order Nonlinear Optical Response in Organic Materials: Theoretical and Experimental Aspects. Chemical Reviews. 94: 243-278. [ Links ]
Dmitriev, V.G., Gurzadyan, G.G., Nikogosyan, D.N. (1991) Handbook of Nonlinear Optical Crystals. Springer, Berlin. [ Links ]
Farrugia, L.J., (1999) WinGX suite for small-molecule single-crystal crystallography. Journal of Applied Crystallography 32: 837-838. [ Links ]
Farrugia, L.J., (1997) ORTEP-3 for Windows - a version of ORTEP-III with a Graphical User Interface (GUI). Journal of Applied Crystallography 30: 565. [ Links ]
Franken, P.A., Hill, A.E., Peters, C.W., Weinreich, G. (1961) Generation of Optical Harmonics. Physical Review Letters 7: 118-119. [ Links ]
Goto, Y., Hayashi, A., Kimura, Y. Nakayama, M. (1991). Second harmonic generation and crystal growth of substituted thienyl chalcones. Journal of Crystal Growth 108: 688-698. [ Links ]
Kerkoc, P., Zgonik, M., Sutter, K., Bosshard, Ch., and Gunter, P. (1990) 4-(N,N-Dimethylamino)-3-acetamidonitrobenzene single crystals for nonlinear-optical applications. Journal of the Optical Society of America B 7:313-319. [ Links ]
Kertcham, R., Jambotkar, D. (1963) The Preparation of and Equilibrium between Substituted α-Phenyl-cis- and trans-cinnamic Acids. Journal of Organic Chemistry 28: 1034:1037. [ Links ]
Muñoz-Flores, B., Santillan, R., Rodríguez, M., Méndez, J.M., Romero, M., Farfán, N., Lacroix, P.G., Nakatani, K., Ramos-Ortíz, G., Maldonado, J.L. (2008) Synthesis, crystal structure and non-linear optical properties of boronates derivatives of salicylideniminophenols Journal of Organometallic Chemistry 693: 1321-1334. [ Links ]
Nalwa, H.S., Miyata, S. (1997) Nonlinear Optics of Organic Molecules and Polymers. 1st Edition. CRC Press., Boca Raton, FL. [ Links ]
Romaniello, P., Lelj, F. (2004) Effects of fluorine atoms on the optical nonlinear response of stilbene derivatives. Journal of Fluorine Chemistry 125: 145-149. [ Links ]
Prasad, P.N., and Williams, D.J. (1991) Introduction to nonlinear optical effects in molecules and polymers. Wiley., New York. [ Links ]
Sheldrick, G.M., (1997) SHELX97, Programs for Crystal Structure Solution and Refinement, University of Gottingen, Gottingen. [ Links ]
Note
This paper is dedicated to Professor Pedro Joseph-Nathan in recognition of his 50 years of outstanding scientific trajectory.














