Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista latinoamericana de química
versión impresa ISSN 0370-5943
Rev. latinoam. quím vol.40 no.3 Naucalpan de Juárez dic. 2012
Ion trap tandem mass spectrometry of C- and N-methyl, benzyl, and prenyl substituted 2-oxopyrrolidinoindolines
Martha S. Morales-Ríosa,*, Eleuterio Burgueño-Tapiab, Nadia A. Pérez-Rojasa, Yolanda Mora-Péreza, Celina Alvarez-Cisnerosa
a Departamento de Química, Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional, Apartado 14-740, México D.F., 07000 México. *Corresponding author. Tel.: +52 55 57477112; Fax: +52 55 57477137; E-mail: smorales@cinvestav.mx.
b Departamento de Química Orgánica, Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, Prolongación de Carpio y Plan de Ayala, Col. Santo Tomás, México, D.F. 11340, México.
Received August 2012.
Accepted October 2012.
ABSTRACT
The electron impact induced fragmentations of C- and N-methyl, benzyl, and prenyl substituted 2-oxopyrrolidinoindolines were studied using an ion trap mass spectrometer (IT-MS). Correlations of characteristic fragment ions of the 2-oxopyrrolidinoindoline skeleton with specific modifications of the substituents around it were supported by stepwise fragmentation MS/MS analysis and accurate mass measurements. The MS3 spectra evidenced the neutral loss of methyl- or benzylisocyanate from the 2-oxopyrrolidine ring, which would result in a rearranged stable quinolinium ion.
Keywords: 2-oxopyrrolidinoindolines, mass spectra, EI-ion trap, MS/MS analysis, quinolinium ion, accurate mass.
RESUMEN
Se estudiaron las fragmentaciones inducidas por impacto electrónico de 2-oxopirrolidinoindolinas sustituidas en C y N por grupos metilo, bencilo, y prenilo usando un espectrómetro de trampa de iones (EM-TI). Las correlaciones de iones-fragmento característicos del esqueleto de 2-oxopirrolidinoindolinas con modificaciones específicas de los sustituyentes alrededor del mismo se sustentaron por análisis de fragmentación gradual EM/EM y mediciones de masa exacta. Los espectros EM3 evidenciaron la pérdida neutra de metil- o bencilisocianato proveniente del anillo de 2-oxopirrolidina que podría resultar en un ión reordenado quinolinio estable.
Palabras clave: 2-oxopirrolidinoindolinas, espectros de masa, trampa de iones-IE, análisis EM/EM, ión quinolinio, masa exacta.
INTRODUCTION
Pyrrolidinoindolines are the basic nuclei of a number of alkaloids that have been isolated from a widespread series of natural sources, including amphibians, plants, and marine organisms (Anthoni et al., 1990; Aygün and Pindur, 2003; Spande et al., 1988; Tokuyama and Daly, 1983). These alkaloids exhibit an impressive array of promising biological properties which includes anticholinesterase activitiy (Rivera-Becerril et al., 2008; Thal et al., 1996; Yu et al., 2010). Owing to their medicinal relevance and structural complexity, pyrrolidinoindoline alkaloids have served as a fertile area for the development of chemical strategies for their synthesis (Crich and Banerjee, 2007; Kim and Movassaghi, 2009; Morales-Ríos and Suárez-Castillo, 2008; Morales-Ríos et al., 2001; Steven and Overman, 2007). A structural survey of this alkaloid family reveals a central cis-fused pyrrolidinoindoline core that in all cases incorporates a quaternary center at the C(3a) site. In addition, N(1), C(3a), and N(8) positions have been shown to incorporate broad variation in substituents.
Mass spectrometry (MS) has proven to be a successful approach for structural elucidation of furoindolines (Clayton and Reed, 1963; Morales-Ríos et al., 2011) and pyrrolidinoindolines (Fales et al., 1970; Rubino and Zecca, 1991; Spande et al., 1988; Spiteller and Spiteller-Friedmann, 1963). In the current study, we were interested in establishing and validating MS fragmentation patterns of a series of 2-oxopyrrolidinoindolines 1a-1f diversely substituted at N(1), C(3a), and N(8) by methyl, benzyl, and prenyl groups (Fig. 1) in order to provide correlations of characteristic fragment ions of the 2-oxopyrrolidinoindoline skeleton with specific modifications of the substituents around it.

MATERIALS AND METHODS
General
Melting point was measured on a Fisher-Johns apparatus and is uncorrected. NMR experiments were performed using Varian Mercury spectrometers working at 300 and 75.4 MHz for 1H and 13C, respectively. Chemical shifts are reported in ppm downfield from tetramethylsilane. IR spectrum was measured with a Perkin-Elmer 16 FPC FT infrared spectrophotometer. All solvents and reagents were purchased in the reagent grade quality and were used without further purification. Solvents for chromatography were purified by distillation. Column chromatography was performed on Silica Gel 60 (230-400 mesh) from Aldrich. 2-Oxopyrrolidinoindolines 1a-1c are known and were synthesized as described (Morales-Ríos et al. 2012) from the corresponding 2-oxofuroindolines 3a-3c by treatment with methylamine.
EI-MS Analysis
The electron impact mass spectrometry (EI-MS) analyses were performed using an ion trap Varian Saturn 2000 spectrometer coupled with a Varian 3800 gas chromatograph. The MS conditions were as follows: transfer line heater, 280 °C; ion source temperature, 220 °C; electron impact ionization (EI) mode; ionization energy, 70 eV; electron multiplier voltage (EMV), 1950 V. The typical mass spectrum was recorded by averaging 1200 scans from m/z 20 to 650 at a scan rate of 1 s/scan. For multistage sequencing MS2/MS3, the compounds were introduced by direct insertion probe and the precursor ions were selected within an isolation width of 2 u. Exact mass measurements for the ions of interest were recorded on a Jeol JMS-GCMate II instrument or on an Agilent LCTOF spectrometer at the UCR Mass Spectrometry Facility, University of California, Riverside, with an error less than ±3 ppm for all ions discussed.
General lactamization procedure
To a solution of the appropriate 2-oxofuroindoline 3a, 3b or 3c (0.65 mmol) in MeOH (20 mL) was added BnNH2 (2.3 equiv, 0.17 mL). The reaction mixture was kept at room temperature for 5-120 h. After this, the solvent was removed under reduced pressure and the residue was suspended in EtOAc (30 mL). The suspension was washed successively with a 5% aq HCl solution (2 x 10 mL) and brine (2 x 10 mL). The organic layer was dried over anhydrous Na2SO4, and concentrated under reduced pressure. The crude product was purified by flash chromatography (7:3 hexane/EtOAc).
1,3a,8-Tribenzyl-5-methoxy-2-oxo-2,3,3a,8a-tetrahydro-8H-pyrrolo[2,3-b] indole (1d). Following the general procedure, a mixture of 3a (250 mg) and BnNH2 in MeOH was refluxed for 96 h to give 1d (219 mg, 71%) as colorless crystals, mp 118-119 °C. TLC: Rf 0.42 (7:3 hexane/ EtOAc); IR (CHCl3) vmax 3010, 2934, 1678, 1602, 1496 cm1. 1H NMR (CDCl3) δ 6.69 (1H, dd, J = 8.6, 2.6 Hz, H6), 6.58 (1H, d, J = 2.5 Hz, H4), 6.39 (1H, d, J = 8.5 Hz, H7), 4.80 (1H, s, H8a), 3.73 (3H, s, OMe), 2.93 and 2.82 (2H, AB, J = 17.2 Hz, H3), N1-Bn: 7.28-7.13 (overlapped, 2Hm, Hp), 6.85 (2H, m, 2Ho), 4.92 and 3.93 (2H, AB, J = 17.5 Hz, CH2), C3a-Bn: 7.28-7.13 (overlapped, 2Hm, Hp), 6.65 (2H, m, 2Ho), 2.80 and 2.66 (2H, AB, J = 13.5 Hz, CH2), N8-Bn: 7.28-7.13 (overlapped, 2Hm, Hp), 6.96 (2H, m, 2Ho), 4.04 and 3.75 (2H, AB, J = 15.7 Hz, CH2); 13C NMR (CDCl3) δ 172.4 (C2), 154.1 (C5), 143.9 (C7a), 136.0 (C3b), 114.2 (C6), 111.0 (C7), 110.2 (C4), 84.9 (C8a), 55.9 (OMe), 51.3 (C3a), 41.9 (C3), N1-Bn 136.5 (Ci), 128.3 (2Cm), 127.6 (2Co), 127.3 (Cp), 43.5 (CH2), C3a-Bn: 136.2 (Ci), 130.1 (2Co), 128.6 (2Cm), 126.9 (Cp), 44.7 (CH2), N8-Bn: 138.7 (Ci), 128.5 (2Cm), 127.5 (2Co), 127.4 (Cp), 54.7 (CH2); EIMS m/z (%) M+• 474 (100), 383 (42), 292 (6), 250 (13), 91 (23).
1-Benzyl-5-methoxy-3a,8-bis(3-methyl-2-buten-1-yl)-2-oxo-2,3,3a,8a-tetrahydro-8H-pyrrolo[2,3-b]indole (1e). Following the general procedure, a mixture of 3b (223 mg) and BnNH2 in MeOH was refluxed for 5 h to give 1e (197 mg, 70%) as pale yellow oil. TLC: Rf 0.20 (7:3 hexane/ EtOAc); IR (CHCl3) νmax 3006, 2972, 1676, 1598, 1494 cm-1;1H NMR (CDCl3) δ 6.69 (1H, dd, J = 8.4, 2.5 Hz, H6), 6.67 (1H, d, J = 2.5 Hz, H4), 6.50 (1H, d, J = 8.5 Hz, H7), 4.62 (1H, s, H8a), 3.75 (3H, s, OMe), 2.81 and 2.72 (2H, AB, J = 17.3 Hz, H3), V1-Bn: 7.32 (2H, m, 2Hm), 7.28 (1H, m, Hp), 7.21 (2H, m, 2Ho), 5.05 and 4.13 (2H, AB, J =15.7 Hz, CH2), C3a-Pre: 5.00 (1H, partially overlapped, ABX, tm, J = 7.4 Hz, CH=), 2.39 and 2.24 (2H, ABX, J = 14.6, 8.0, 7.4 Hz, CH2), 1.70 (3H, s, Me), 1.48 (3H, s, Me); N8-Pre: 5.10 (1H, partially overlapped, ABX, tm, J = 7.0 Hz, CH=), 3.74 and 3.60 (2H, ABX, J = 15.8, 7.7, 7.0 Hz, CH2), 1.59 (3H, s, Me), 1.74 (3H, s, Me); 13C NMR (CDCl3) δ 173.0 (C2), 154.1 (C5), 143.5 (C7a), 137.7 (C3b), 113.6 (C6), 111.1 (C7), 109.8 (C4), 85.5 (C8a), 55.8 (OMe), 50.2 (C3a), 41.5 (C3), N1-Bn: 136.3 (Ci),128.5 (2Cm), 127.3 (2Co), 127.2 (Cp), 43.4 (CH2), C3a-Pre: 135.9 (C=), 118.4 (CH=), 37.3 (CH2), 25.9 (Me), 17.9 (Me), N8-Pre: 135.1 (C=), 120.8 (CH=), 48.8 (CH2), 25.6 (Me), 17.6 (Me); EIMS m/z (%) M+• 430 (100), 362 (17), 293 (67), 160 (22), 91 (10).
1,8-Dibenzyl-5-methoxy-3a-(3-methyl-2-buten-1-yl)-2-oxo-2,3,3a,8a-tetrahydro-8H-pyrrolo[2,3-b]indole (1f). Following the general procedure, a mixture of 3c (236 mg) and BnNH2 in MeOH was refluxed for 120 h to give 1f (244 mg, 83%) as pale yellow oil. TLC: Rf 0.48 (7:3 hexane/ EtOAc); IR (CHCl3) vmax 3016, 2934, 1678, 1598 cm-1; 1H NMR (CDCl3) δ 6.67 (1H, overlapped, H4), 6.64 (1H, partially overlapped, dd, J = 8.4, 2.6 Hz, H6), 6.38 (1H, d, J = 8.0 Hz, H7), 4.70 (1H, s, H8a), 3.74 (3H, s, OMe), 2.78 (2H, s, H3), N1-Bn: 7.32-7.22 (overlapped, 2Hm, Hp), 7.03 (2H, m, 2Ho), 5.04 and 3.93 (2H, AB, J = 15.6 Hz, CH2), C3a-Pre: 4.91 (1H, ABX, tm, J = 7.3 Hz, CH=), 2.29 and 2.13 (2H, ABX, J = 14.5, 8.1, 7.3 Hz, CH2), 1.66 (3H, s, Me), 1.39 (3H, s, Me), N8-Bn: 7.32-7.22 (overlapped, 2Hm, Hp), 7.12 (2H, m, 2Ho), 4.33 and 4.18 (2H, AB, J = 16.2 Hz, CH2); 13C NMR (CDCl3) δ 173.2 (C2), 154.1 (C5), 143.9 (C7a), 137.0 (C3b), 113.5 (C6), 110.3 (C7), 110.1 (C4), 86.4 (C8a), 55.9 (OMe), 50.1 (C3a), 41.8 (C3), N1-Bn: 136.2 (Ci, 128.6 (2Cm), 127.6 (2Co), 127.4 (Cp), 43.8 (CH2), C3a-Pre: 136.0 (C=), 118.3 (CH=), 37.4 (CH2), 25.9 (Me), 18.0 (Me), N8-Bn: 138.7 (Ci, 128.5 (2Cm), 127.3 (Cp), 127.2 (Co), 55.3 (CH2); EIMS m/z (%) M+• 452 (100), 383 (41), 292 (3), 250 (7), 91 (2).
RESULTS AND DISCUSSION
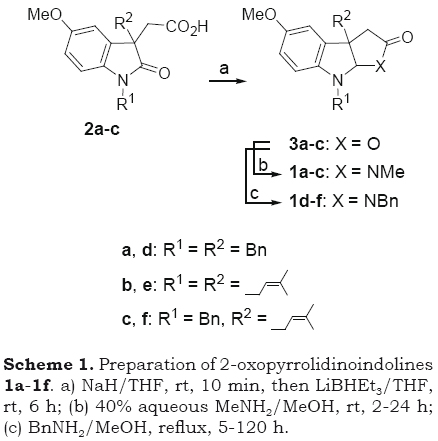
The preparation of 2-oxopyrrolidinoindoli-nes 1a-1f was achieved in two steps from the sodium salt of 2-(1,3-dialkyl-2-oxo-3-indolyl)acetic acids 2a-2c. The reductive cyclization of 2a-2c with LiBHEt3 gave the corresponding 2-oxofuroindolines 3a-3c. Stirring MeOH solutions of 3a-c with methylamine at room temperature for 2-24 h or with benzylamine in boiling methanol for 5-120 h gave the expected 2-oxopyrrolidinoindolines 1a-1f in a combined yield of 40-65% for the two-step process (Scheme 1).
The electron impact mass spectra (EI-MS) of compounds 1a-1f (Table 1) were complemented by tandem mass spectrometry (MS/MS). This technique involves the isolation of a specific ion, and the subsequent fragmentation thereof. Figure 2 illustrates MS1 and MS2 spectra obtained from 1a-1f. In single IT-MS mode (Fig. 2, left side), the base peaks corresponding to M+• were observed along with several fragment ions. These spectra are very alike, exhibiting common peaks that correspond to losses of 91 Da for 1a and 1d, and of 69 Da for 1b, 1c, 1e and 1f. The accurate mass measurement and the stepwise fragmentation MS2 analysis of 1a-1f (Fig. 2, right side) revealed that such losses involved the cleavage of the benzyl or prenyl moieties (Table 1). The EI-MS of compounds 1a or 1d acquired in MS3 mode revealed that the fragment ions were very similar from those of 1c or 1f, respectively, evidencing that the base peaks in the MS2 mode (Fig. 2, right side) were formed from loss of the angular C(3a)-substituent moiety. Whereas, compounds 1b and 1e, both characterized by the presence of two prenyl groups at positions C(3a) and N(8), exhibit significant peaks in the MS2 mode at m/ z 285 and 217 for 1b and at m/z 362 and 293 for 1e attributed to the loss of one or two of the prenyl groups from the molecular ions (Fig. 2, right side). The elemental compositions of these ions were confirmed using accurate mass measurements, which afforded relative errors within the range -2.7 to 1.4 ppm (Table 2).
Continuing with the fragmentation process of 1a-1f, the cleavage of the lactam ring give rise to m/ z 160 ion in the MS3 spectra of 1b and 1e and to m/ z 250 ion in the MS3 spectra of 1a, 1c, 1d, and 1f (Scheme 2). These ions could derived from neutral loss of methyl- or benzylisocyanate (MeN=C=O or BnN=C=O), which are concurrent with the elimination of the prenyl group at N8 in the case of 1b and 1e. The m/ z 160 and 250 are probably formed with simultaneous rearrangement to the stable ring-expanded quinolinium ions, whose plausible structures are shown in Scheme 2. The accurate mass measurements (Table 2) confirmed the composition of the fragments of interest, i.e. m/z 250.1233 for 1a (calcd for C17H16NO+ 250.1232) and m/z 160.0765 for 1b (calcd for C10H10NO + H+ 160.0762).

Although the dominant fragmentation pathway for 1a, 1c, 1d and 1f includes the consecutive losses of R2• and X=C=O from M+• to give ion II (Fig. 3, path i), an alternative fragmentation route of ion I proceeds via elimination of Bn• to give ion III (path ii) nevertheless in very low relative intensity (≤ 3%, Table 1). In contrast, elimination of Pre• from ion I (path ii) is the only one fragmentation pathway for 1b and 1e giving [M-Pre-Pre + H]+ ion III in 24% and 67%, respectively (Table 1).
CONCLUSIONS
The present study provided insight into the fragmentation patterns of diversely substituted 2-oxopyrrolidinoindolines 1a-1f. The multiple-stage capability of the IT-MS together with accurate mass measurements on high-resolution instruments were invaluable for establishing fragmentation pathways, and greatly aided in proposing fragment ion structures.
ACKNOWLEDGEMENTS
This work was supported by Conacyt-México (grants 139736 and 168066).
REFERENCES
Anthoni, U., Nielsen, P.H., Pereira, M., Christophersen, C. (1990) Bryozoan secondary metabolites: a chemotaxonomical challenge. Biochemistry & Molecular Biology 96B: 431-437. [ Links ]
Aygün, A., Pindur, U. (2003) Chemistry and biology of new marine alkaloids from the indole and annelated indole series. Current Medicinal Chemistry 10: 1113-1127. [ Links ]
Clayton, E., Reed, R.I. (1963) The mass spectra of physostigmine and some related compounds. Tetrahedron 19: 1345-1357. [ Links ]
Crich, D., Banerjee, A. (2007) Chemistry of the hexahydropyrrolo[2,3-b]indoles: configuration, conformation, reactivity, and applications in synthesis. Accounts of Chemical Research 40: 151-161. [ Links ]
Fales, H.M., Lloyd, H.A., Milne, G.W.A. (1970) Chemical ionization mass spectrometry of complex molecules. II. Alkaloids. Journal of the American Chemical Society 92: 1590-1597. [ Links ]
Kim, J., Movassaghi, M. (2009) Biogenetically inspired syntheses of alkaloid natural products. Chemical Society Reviews 38: 3035-3050. [ Links ]
Morales-Ríos, M.S., López-Camacho, P.Y., Jacobo-Cabral, C.O., Pérez-Rojas, N.A., Trujillo-Serrato, J.J., Burgueño-Tapia, E., Suárez-Castillo, O.R., Joseph-Nathan P. (2011) Unimolecular rearrangements of ketene-0,0-acetals and fragmentations occurring in the gas phase. Journal of Mass Spectrometry 46: 489-495. [ Links ]
Morales-Ríos, M.S., Rivera-Becerril, E., López Camacho, P.Y., Pérez-Rojas, N.A., Suárez-Castillo, O. R. (2012) Preparation of 0-methyl substituted 2-oxofuro- and 2-oxopyrrolidinoindolines by reductive lactonization of oxindol-3-ylacetic acids. Natural Product Communications 7: 1445-1451. [ Links ]
Morales-Ríos, M.S., Suárez-Castillo O.R. (2008) Synthesis of marine indole alkaloids from Flustra foliacea. Natural Product Communications 3: 629-642. [ Links ]
Morales-Ríos, M.S., Suárez-Castillo, O.R., Trujillo-Serrato, J.J., Joseph-Nathan P. (2001) Total syntheses of five indole alkaloids from the marine bryozoan Flustra foliacea. Journal of Organic Chemistry 66: 1186-1192. [ Links ]
Rivera-Becerril, E., Joseph-Nathan, P., Pérez-Alvarez, V.M., Morales-Ríos, M.S. (2008) Synthesis and biological evaluation of (-)- and (+)-debromoflustramine B and its analogues as selective butyrylcholinesterase inhibitors. Journal of Medicinal Chemistry 51: 5271-5284. [ Links ]
Rubino, F.M., Zecca, L. (1991) Retrosynthetic fragmentation in the fast atom bombardment mass spectra of eserine and some related compounds. Organic Mass Spectrometry 26: 961-966. [ Links ]
Spande, T. F., Edwards, M.W., Pannell, L.K., Daly, J.W. (1988) Pseudophrynamine A: an unusual prenyl pyrrolo[2,3-b]indole ester from an australian frog, Pseudophryne coriácea (Myobatrachidae). Journal of Organic Chemistry 53: 1222-1226. [ Links ]
Spiteller, G., Spiteller-Friedmann, M. (1963) Key fragments in alkaloid mass spectra. Tetrahedron Letters 147-152. [ Links ]
Steven, A., Overman, L.E. (2007) Total synthesis of complex cyclotryptamine alkaloids: stereocontrolled construction of quaternary carbon stereocenters. Angewandte Chemie International Edition 46: 5488-5508. [ Links ]
Thal, L.J., Schwartz, G., Sano, M., Weiner, M., Knopman, D., Harrell, L., Bodenheimer, S., Rosser, M., Philpot, M., Schor, J., Goldberg, A. (1996) A multicenter double-blind study of controlled-release physostigmine for the treatment of symptoms secondary to Alzheimer's disease. Neurology 47: 1389-1395. [ Links ]
Tokuyama, T., Daly, J.W. (1983) Steroidal alkaloids (batrachotoxins and 4p-hydroxybatrachotoxins), "indole alkaloids" (calycanthine and chimonanthine) and a piperidinyldipyridin. Tetrahedron 39: 41-47. [ Links ]
Yu, Q.-s., Holloway, H.W., Luo, W., Lahiri, D.K., Brossi, A., Greig, N.H. (2010) Long-acting anticholinesterases for myasthenia gravis: synthesis and activities of quaternary phenylcarbamates of neostigmine, pyridostigmine and physostigmine. Bioorganic & Medicinal Chemistry 18: 4687-4693. [ Links ]
Note
This paper is dedicated to Professor Pedro Joseph-Nathan in recognition of his 50 years of outstanding scientific trajectory.














