Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista latinoamericana de química
versión impresa ISSN 0370-5943
Rev. latinoam. quím vol.40 no.1 Naucalpan de Juárez abr. 2012
Acute toxicity and decreased peristalsis in mice caused by Taxodium mucronatum and Acacia farnesiana extracts
Steve de la Cruz, Eduardo Rodríguez, Hortencia N. Dávalos, Adela Astudillo-Vázquez*
Departamento de Biofísica, Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, Carpio y Plan de Ayala s/n, Casco de Santo Tomás, CP 11340, México, Distrito Federal, México. *Corresponding author: adela_av@yahoo.com.mx, adelaav@unam.mx.
Received May 2012
Accepted September 2012
Abstract
Diarrheal diseases remain a health problem in Mexico and the world. Taxodium mucronatun Ten. (Taxodiaceae) (Ahuehuete, Sabino) and Acacia farnesiana (L.) Will. (Leguminosae) (Huizache) are popularly used in the treatment of diarrhea. The aim of this work was to determine, in vivo, the lethal media doses and effect on intestinal propulsion of aqueous and ethanol extracts from leaves and small stems in mice. The lethal media doses were estimated using the Lorke method, modified by the Reed-Müench approach, and probit units; the results indicated a weak acute toxicity level. The effects on intestinal propulsion were tested using the charcoal meal method and we found that the extracts inhibited the movement of the gastrointestinal content. The data explain in part the medicinal use of T. mucronatum and A. farnesiana, and support their use as antidiarrheal agents.
Key words: Taxodium mucronatum, Ahuehuete, Acacia farnesiana, Huizache, lethal media dose, antidiarrheal.
Resumen
Las enfermedades diarreicas siguen siendo un problema de salud en México y el mundo. Taxodium mucronatum Ten. (Taxodiaceae) (Ahuehuete, Sabino) y Acacia farnesiana (L.) Will. (Leguminosae) (Huizache), se usan popularmente en el tratamiento de la diarrea. El objetivo de este trabajo fue determinar, in vivo, la dosis letal media y el efecto sobre la propulsión intestinal en ratones, de los extractos acuoso y etanólico de hojas y tallos pequeños de T. mucronatum y de A. farnesiana. Las dosis letales medias se estimaron por el método de Lorke modificado tomando en consideración los planteamientos de Reed-Müench y el uso de unidades probitas, los resultados indicaron un débil nivel de toxicidad aguda de estas especies. El efecto sobre la propulsión intestinal se probó utilizando el método de carbón activado, se encontró que ambos extractos inhibieron el avance del contenido gastrointestinal. Los resultados contribuyen a explicar el uso medicinal del Ahuehuete y del Huizache como agentes antidiarreicos.
Palabras clave: Taxodium mucronatum, Ahuehuete, Acacia farnesiana, Huizache, dosis letal media, antidiarreico.
Introduction
Diarrheal diseases continue to be a cause of morbidity and mortality worldwide (WHO-SIS, 2009; WHO, 2009; UNICEF, 2009). In Mexico, in 2005, infectious intestinal diseases (including diarrhea) were included in the top 20 causes of death in men, women, and people over 65 years of age (SS, 2005).
Taxodium mucronatum Ten. (Taxodiaceae) (Ahuehuete, Sabino) (synonymy with Taxodium montezumae, Taxodium mexicanum). Ahuehuete is a tree that is mentioned in the Florentine Codex. Its name is a nahuatl word that translates to "old man from the water", it is a millenary species linked to Mexican history. The Ahuehuete is 20 to 30 m high, its trunk is thick and frequently divided from the base, it has a brown-reddish bark. The branches form a wide crown, with hanging twigs and linear sheets (Rzedowski and Rzedowski, 1991). In Mexican traditional medicine, it is used to treat diarrhea as well as skin diseases, sores, hemorroids, burns (Argueta, 1994). It contains biflavones (Ishratullah et al., 1978), amentoflavone and its derivatives (Pan et al., 2005), flavonoids, quercetin glucoside, and a diterpenoid (Argueta, 1994).
Acacia farnesiana (L.) Willd. (Leguminosae) (Huizache, Cascalote, Colita, Espino blanco), (synonymy with Acacia aciculans, Mimosa farnesiana and Vachellia farnesiana). Huizache is a tree or shrub, 2 to 5 m in height, its trunk is highly branched, stipules has shaped thorns, short-petioled leaves, 2 to 6 cm long (Rzedowski and Rzedowski, 1990), the flowers are fragrant, yellow, and its fruits are cylindrical dark pods; this species is distributed throughout the country, mainly in arid and semiarid regions (Argueta, 1994). A. farnesiana is used in the Mexican traditional medicine for the treatment of diarrhea, dysentery, diabetes, wounds, cough, and typhoid (Argueta, 1994). A. farnesiana contains 7, 3' dihydroxy-4' methoxy flavone (farnisin) (Sahu et al., 1998), cyanogens (linamarin and lotaustralin) (Seigler et al., 1979), tannins (Wassel et al., 1990), kaempferol and its derivatives (El-Negoumy et al., 1982), naringenin 7-O-β-(4"6"-digalloylglucopyranoside), quercetin 7-O-β-(6"-galloylglucopyranoside), myricetin 7-O-β-(6"-galloylglucoside), kaempferol 7-(6" galloylglucoside) (Barakat et al., 1999), among other compounds.
Both species, Taxodium mucronatum and Acacia farnesiana, are used to treat diarrhea, in aqueous decoction from leaves and small stems.
There are drugs that have proved to be effective to treat diarrhea (loperamide, diphenoxylate); however, they have shown adverse effects (Burks, 1998; Guststein and Alkil, 2003; Jafri and Pasricha, 2003) and they are not readily available for the majority of the population due to their price. For these reasons, it is necessary to undertake the study of plants whose ethnobotanic references point them out as species with action against diarrhea. It is imperative to explore their toxicity degree given that not all species are innocuous. Moreover, considering that the common factor in diarrheas is an increased intestinal motility, research on this issue must be done. The aim of the present work was to determine the acute degree of toxicity, as well as the effect on the gastrointestinal motility in mice of two medicinal plants currently used for the treatment of diarrhea in Mexico.
Material and methods
Plant material. Leaves and small stems from Taxodium mucronatum Ten. were collected in Mexico City and leaves and small stems from Acacia farnesiana (L.) Willd. in Hidalgo State, Mexico. These specimens were taxonomically identified by Biol. Alfredo Patiño-Siciliano (Departamento de Botánica, Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional). Voucher specimens were deposited in the IEB Herbarium (Centro Regional del Bajío, Instituto de Ecología, A. C., Pátzcuaro, Mich.) [T. mucronatum: IEB 007069; A. farnesiana: IEB 071574].
Animals. Swiss albino mice (20-25 g, NIH) maintained under standard conditions and fed with standard diet and water ad libitum were used in all experiments. The animals were treated in compliance with the national and international recommendations (Olfert et al., 1993; SS, 1999; Lomelí, 2002), routinely used in our laboratory.
Materials. Loperamide (Sigma-Aldrich, Inc., St. Louis, MO, USA), Arabic gum (Química Meyer), charcoal meal (Hycel Mexico). Other chemicals were analytical grade.
HPLC analysis
Sample: Quercetin, aqueous and ethanol extracts of leaves and twigs of T. mucronatum and A. farnesiana. Chromatographic conditions: Column Symmetry C18 5 µm (4.6 × 250 mm). DAD detector at 270 nm. Oven temperature 30°C. Injection volume of 10 µL. Mobile phase: A: Phosphoric acid 3% (v/v). B: MeOH; C: ACN. Elution gradient: initio 100% A; 10 min, 85% A, 15% C; 30 min, 70% A, 10% B, 20% C; 40 min, 10% A, 15% B, 75% C; 55 min, 5% A, 15% B, 80% C; 56 min, 100% A; 66 min 100% A. Flow: 1 mL/min).
Extract preparation
Dried and powdered leaves and small stems of each plant were Soxhlet extracted with distilled water. The extract was filtered through gravity and concentrated in a rotary evaporator Buchi RE-111, and finally exposed to an air current at room temperature (22 ± 2 °C), until a dark brown semisolid mass was formed (yield 16.8% dry weight of T. mucronatum and 25.4% dry weight of A. farnesiana). Ethanol extracts were prepared in ethanol (96%) maceration until a dark green semisolid mass was formed (yield 8.7% dry weight of T. mucronatum and 16.8% dry wt. of A. farnesiana).
Plant extracts were freshly prepared each time and administered at a volume of 0.1 mL/10 g body weight for all the pharmacological tests.
Acute toxicity test
The following was administered i.p. to groups of three mice: isotonic saline solution to the control group. The test group dosing was given according to that established by Lorke (1983). The 48-hour mortality was registered and the survivors were kept in observation for the next 15 days.
Taking into account the Lorke method (1983), modified by the Reed-Müench approach (Pouillot et al., 2003), and the use of probit analysis. The equation to determine the lethal media doses (LD50):
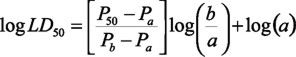
[a: dose < 50% mortality; b: dose > 50% mortality; P50: probit units at 50% mortality; Pa: probit units at dose a; Pb: probit units at dose b].
Intestinal motility test
Mice were starved for 24 h prior to the experiment, but were allowed free access to water. Extract was (1-300 mg/kg) (Astudillo-Vázquez et al., 2008) orally administered to animals (n = 10). Loperamide (5 mg/kg, po) was given to animals in the reference group while control animals received isotonic saline solution. Thirty minutes after drug administration, a 10% charcoal suspension in a 5% aqueous suspension of arabic gum was po administered to each animal. After thirty minutes, the animals were killed by cervical dislocation and the entire length of the intestine (from the pylorus to the cecum) was removed carefully. The distance travelled by the charcoal plug in the intestine (A) and the total length of the intestine (B) was measured (Williamson et al, 1996). The percentage of intestinal transit was calculated for each mouse as (A/B) × 100.
Statistical analysis
The results were statistically evaluated using a one-way ANOVA followed by Dunnett's t test (p < 0.05).
Results and discussion
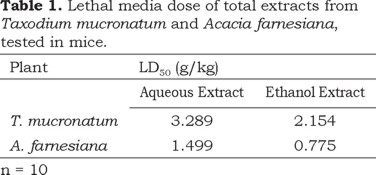
The results obtained for the LD50 from the extracts of T. mucronatum and A. farnesiana (Table 1) show that they are weakly toxic according to classifications based on this parameter (Déciga-Campos et al., 2007). This means that they do not induce immediate toxicity in the tested experimental conditions. Also the pharmacologically active doses were lower than lethal media doses obtained. It has been reported that the genus Acacia is included in the wide group of plants that contain cyanogens (Seigler et al., 1979). LD50 results suggest that A. farnesiana does not have or has low concentrations of cyanogen compounds.
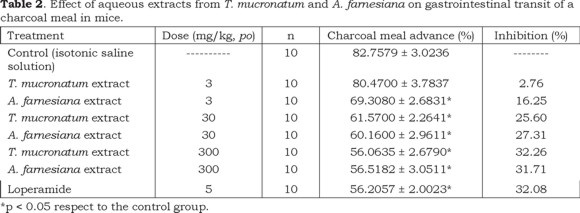
The results show that the extracts of both the Ahuehuete and the Huizache were able to diminish the intestinal motility; with all the extracts a rising tendency of the inhibitory effect on the gastrointestinal motility was observed when the doses were increased (Tables 2 and 3). Inhibition percentages of peristaltic motion at the highest dose tested (300 mg/kg) for both extracts were 30% higher as compared to the control group. This level is considered appropriate for the total extract, considering that the population consumes medicinal teas of both species to treat diarrhea. In this study, the charcoal meal method was selected to follow the displacement of the gastrointestinal content because reduction of gastrointestinal motility is one mechanism by which many antidiarrheal agents can act (Akah et al., 1999; Astudillo-Vázquez et al., 2009); this may be the case of both Taxodium mucronatum and Acacia farnesiana. Phytochemical studies of the Ahuehuete (Ishratullah et al., 1978, Argueta, 1994; Pan et al., 2005) and the Huizache (Barakat et al., 1999), have detected that they contain flavonoids among other compounds.
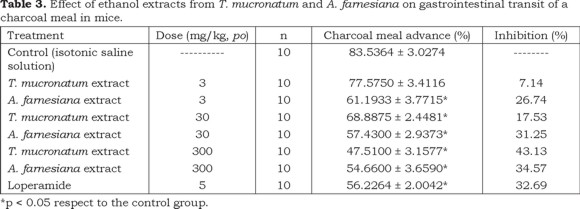
One of the flavonoids present in A. farnesiana is kaempferol (El-Negoumy et al., 1982), which is a compound with antispasmodic activity in guinea pig isolated ileum (Trute et al., 1997) and its mechanism is exerted through calcium mobilization (Capasso et al., 1991). Furthermore, the mucilage that contains A. farnesiana gives body and volume to feces by favoring water absorption, which may contribute to decrease the amount of liquid feces.
On the other hand, quercetin derivatives are present in A. farnesiana (Barakat et al., 1999) and T. mucronatum (Argueta, 1994), this flavonoid inhibits the contractile responses in guinea pig isolated ileum (Lutterodt, 1989), probably by reducing the available calcium concentration (Capasso et al., 1991). More research is needed to elucidate the mechanism by which the extracts work. Of course, it is also necessary to isolate the active compounds. HPLC analysis of the extracts studied did not indicate the presence of quercetin, but it showed other aromatic compounds, perhaps biflavonoids, however this cannot be stated conclusively.
Conclusions
Our results suggest that the extracts obtained from the species studied do not induce immediate toxicity in the tested experimental conditions.
The obtained data present evidence that supports the use of T. mucronatum and A. farnesiana as antidiarrheal agents in Mexican traditional medicine.
Acknowledgements
The authors thank Biol. Alfredo Patiño Siciliano for the taxonomic identification of the plant material. Thanks to Prof. Daniel Villegas Estrada for mathematical assistance. A. Astudillo-Vázquez is thankful for the COFAA-IPN grant. Sponsors: SIP-IPN (20121460) through partial support.
References
Akah, P.A., Aguwa, C.N., Agu, R.U. (1999) Studies on the antidiarrhoeal properties of Pentaclethra macrophylla leaf extracts. Phytoterapy Research 13 : 292-295. [ Links ]
Argueta, A. (Coordinador general). (1994) Atlas de las plantas de la medicina tradicional mexicana. Tomos I y II. Instituto Nacional Indigenista. Mexico City. Mexico. pp. 61-62, 833-834. [ Links ]
Astudillo-Vázquez, A., Dávalos, H., De Jesús, L., Herrera, G., Navarrete, A. (2008) Investigation of Alternanthera repens and Bidens odorata on gastrointestinal disease. Fitoterapia 79 : 577-580. [ Links ]
Astudillo-Vázquez, A., Mata, R., Navarrete, A. (2009) El reino vegetal, fuente de agentes antiespasmódicos gastrointestinales y antidiarreicos. Revista Latinoamericana de Química 37 : 7-44. [ Links ]
Barakat, H.H., Souleman, A.M., Hussein, S.A.M., Ibrahiem, O.A., Nawwar, M.A.M. (1999) Flavonoid galloyl glucosides from the pods of Acacia farnesiana. Phytochemistry 51 : 139-142. [ Links ]
Burks, T.F. (1998) Gastrointestinal drugs. Chapter 62. In: Human pharmacology. Molecular to clinical. Brody, T.M., Larner, J., Minneman, K.P. (editors). 3rd edition. Mosby. St. Louis Missouri, USA. pp. 827-841. [ Links ]
Capasso, A., Pinto, A., Sorrentino, R., Capasso, F. (1991) Inhibitory effects of quercetin and other flavonoids on electrically-induced contractions of guinea pig isolated ileum. Jounal of Ethnopharmacology 34 : 279-281. [ Links ]
Déciga-Campos, M., Rivero-Cruz, I., Arriaga-Alba, M., Castañeda-Corral, G., Angeles-López, G.E., Navarrete, A., Mata, R. (2007) Acute toxicity and mutagenic activity of Mexican plants used in traditional medicine. Journal of Ethnopharmacology 110 : 334-342. [ Links ]
El-Negoumy, S.I., El-Ansari, M.A. (1982) Kaempferol 7-galloylglucosa from Acacia farnesiana. Egyptian Journal of Chemistry 24 : 471-473. [ Links ]
Gutstein, H.B., Akil, H. (2003) Analgésicos opioides. In: Goodman & Gilman. Las bases farmacológicas de la terapéutica. Hardman, J.G, Limbird, L.E., Goodman Gilman A. (eds).10th Edition. Volume I. Mexico City. Mexico. pp. 577-628. http://sinais.salud.gob.mx/ [ Links ]
Ishratullah, K.H., Rahman, W., Okigawa, M., Kawano, N. (1978) Biflavones from Taxodium mucronatum. Phytochemistry 17 : 335. [ Links ]
Jafri, S., Pasricha, P.J. (2003) Fármacos usados para diarrea, estreñimiento y enfermedad inflamatoria intestinal; fármacos usados para enfermedades biliar y pancreática. In: Goodman & Gilman. Las bases farmacológicas de la terapéutica. Hardman, J.G., Limbird, L.E., Goodman Gilman A. (eds).10th Edition. Volume I. Mexico City. Mexico. pp. 1051-1072. [ Links ]
Lomelí, C. (ed.) (2002) Guía para el cuidado y uso de los animales de laboratorio. Institute of Laboratory Animal Resources Council, Commission on Life Sciences, National Research Council. Mexico: Academia Nacional de Medicina. pp. 25-55. [ Links ]
Lorke, D. (1983) A new approach to practical acute toxicity testing. Archives of Toxicology 54 : 275-287. [ Links ]
Lutterodt, G.D. (1989) Inhibition of gastrointestinal release of acetylcholine by quercetin as a possible mode of action of Psidium guajava leaf extracts in the treatment of acute diarrhoeal disease. Journal of Ethnopharmacology 25 : 235-247. [ Links ]
Olfert, E. D., Cross, B. M., McWilliam, A. A. (eds.). (1993) Guide to the care and use of experimental animals. Vol 1, Ontario, Canada: Canadian Council on Animal Care. 211 p. [ Links ]
Pan, X., Tan, N., Zeng, G., Zhang, Y., Jia, R. (2005) Amentoflavone and its derivatives as novel natural inhibitors of human cathepsin B. Bioorganic & Medicinal Chemistry 13 : 5819-5825. [ Links ]
Pouillot, R., Grilló, M.J., Alabart, J.L., Garin-Bastuji, B., Blasco, J.M. (2003) Statistical procedures for calculating the residual virulence of Brucella abortus strain 19(S19) and Brucella melitensis strain Rev 1 vaccines in mice: theoretical basis and practical applications. Scientific and Technical Review 22:1051-1063. [ Links ]
Rzedowski, J., Rzedowski, G.C. (1990) Flora fanerogámica del Valle de México. Vol. III. Instituto de Ecología. Mexico p. 405. [ Links ]
Rzedowski, J., Rzedowski, G.C. (1991) Flora fanerogámica del Valle de México. Vol. I. Escuela Nacional de Ciencias Biológicas. Instituto Politécnico Nacional. Mexico, pp. 71-72. [ Links ]
Sahu, N.P., Achari, B., Banerjee, S. (1998) 7,3'-dihydroxy-4'-methoxyflavone from seeds of Acacia farnesiana. Phytochemistry 49 : 1425-1426. [ Links ]
Seigler, D.S., Conn, E.E., Dunn, J.E., Janzen, D.H. (1979) Cyanogenesis in Acacia farnesiana. Phytochemistry 18 : 1389-1390. [ Links ]
SS, Secretaría de Salud (1999) Norma Oficial Mexicana. Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio (NOM-062-ZOO-1999). Mexico: Diario Oficial de la Federación. [ Links ]
SS, Secretaría de Salud (2005) Principales causas de mortalidad general, en hombres, en mujeres y en edad posproductiva (65 años y más). Mexico. [ Links ]
Trute, A., Gross, J., Mutschler, E., Nahrstedt, A. (1997) In vitro antispasmodic compounds of the dry extract obtained from Hedera helix. Planta Medica 63 : 125-129. [ Links ]
UNICEF, Fondo de las Naciones Unidas para la Infancia (2009) Estado mundial de la infancia 2009. Salud materna y neonatal: Situación actual. http://www.unicef.org/ [ Links ]
Wassel, G.M., Abd-El-Wahab, S.M., Aboutabl, E.A., Ammar, N.M., Afifi, M.S. (1990) Study of phenolic constituents and tannins isolated from Acacia nilotica (L.) Willd and Acacia farnesiana (L.) Willd growing in Egypt. Herba Hung 29 : 43-49. [ Links ]
WHO, Organización Mundial de la Salud (2009). Estadísticas Sanitarias Mundiales. WHO. p. 53. [ Links ]
WHOSIS, World Health Statistics Information System (2009) http://www.who.int/whosis/ [ Links ]
Williamson, E. M., Okpako, D.T., Evans, F. J. (1996) Selection, preparation and pharmacological evaluation of plant material. Pharmacological methods in Phytotherapy Research. John Wiley and Sons. Chichester. p. 28. [ Links ]














