Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista latinoamericana de química
Print version ISSN 0370-5943
Rev. latinoam. quím vol.39 n.1-2 Naucalpan de Juárez 2011
Ca2+, Mg2+ OR Fe2+ ion-exchanged cancrinite-type zeolites as possible hypoglycemiant agents
Vanessa Cisneros, Freddy Ocanto, Carlos F. Linares*
* Laboratorio de Catálisis y Metales de Transición. Facultad de Ciencias y Tecnología. Departamento de Química. Universidad de Carabobo. Valencia. Edo. Carabobo. Venezuela. Apartado Postal 3336, email: clinares@uc.edu.ve.
Received September 2011.
Accepted November 2011.
ABSTRACT
A nitrate-sodium cancrinite-type zeolite was synthesized and exchanged with calcium (II), magnesium (II) and iron (II) cations. These exchanged zeolites were previously characterized by XRD, BET specific surface area, chemical analysis and FT-IR. XRD confirmed only the presence of the cancrinite phase. Other phases, such as sodalite, were not identified. Different amounts of these ion-exchanged zeolites were put in contact with a glucose solution during variable time. Results showed that these ion-exchanged cancrinites were active for the glucose adsorption, especially those exchanged with Fe; while the Ca2+, Mg2+ and Na+ cancrinites displayed an adsorption capacity much lower than the Fe2+ cancrinite. Moreover, the kinetic studies showed that the glucose adsorption capacity by using these modified cancrinites, it is affected by the glucose concentration in the reaction medium.
Key words: cancrinite, hypoglycemiant agents, diabetes, cationic exchange.
RESUMEN
Se sintetizó una zeolita tipo cancrinita sódica y nitrada, y se intercambió con cationes calcio (II), magnesio (II) y hierro (II). Estas zeolitas se caracterizaron, previamente, por difracción de rayos X, área superficial específica BET, análisis químico e infrarrojo con transformada de Fourier. Los datos de DRX confirmaron únicamente la presencia de la fase cancrinita. Otras fases, como la sodalita, no fueron detectadas. Estas zeolitas intercambiadas fueron puestas en contacto con una solución de glucosa a diferentes masas y tiempos de contacto. Los resultados señalaron que estas cancrinitas modificadas fueron activas para la adsorción de glucosa, especialmente las zeolitas intercambiadas con Fe, mientras que las cancrinitas intercambiadas con Ca2+, Mg2+ y Na+ mostraron una capacidad de adsorción mucho más baja que la cancrinita de Fe. Además, los estudios cinéticos demostraron que la capacidad de adsorción de glucosa por estas zeolitas es afectada por la concentración de glucosa en el medio de reacción.
Palabras clave: cancrinita, agentes hipoglicemiantes, diabetes, intercambio catiónico
INTRODUCTION
Type-II diabetes or Diabetes mellitus is characterized by the inadequate use of the insulin by human organism; therefore, the glucose levels in the blood are not normal. This type of diabetes can be controlled by diets, exercises and a low ingestion of sugar or carbohydrates to control the glycemia levels. However, when the sugar levels in the blood are very high, several drugs should be frequently administered in order to regulate the glucose concentration in the organism. These drugs are composed of sulfonylureas, which stimulate to the pancreas to produce insulin (White et al. 1959). These compounds are very effective but when they are used for long time, their effectiveness is lost. Then, new treatments based on the insulin administration should be started.
Nowadays, new alternatives based on zeolites can also be considered. Because of their well-know adsorption properties, they can reduce the glucose concentration in blood. Concepción-Rosabal et al. (1997) tested exchanged natural clinoptilolites with diverse cations, and they found that these modified zeolites could be used as glucose adsorbents. However, the heterogeneity, normally present in the natural zeolite ores, could be an undesirable element. Therefore, synthetic-instead of natural- zeolites could be considered. Previously, Sherman and Chao (1989) had tested zeolites X and Y modified with K+ and Ca2+. They found that those zeolites were able to separate different saccharides. Likewise, Heper et al. (2007) established studies of adsorption kinetics at 50°C by Na+, NH4+, Ca2+ and Mg2+ forms of zeolite Y contacted with aqueous solutions containing glucose and/or fructose. They found a selective adsorption of the glucose or fructose depending on the cationic form. These cations form complexes with the hydroxyl group of the adsorbed sugar, leading to a selective adsorption according to the orientation of the hydroxyl group (Nobre et al. 2009)
On the other hand, Buttersack et al (1993) studied dealuminated zeolites Y and found that Si/Al ratio had a significant influence on the sugar's selective adsorption.
Taking into account these results, it is possible to consider the use of zeolites for adsorption of glucose.
In that sense, our group has tested cancrinite-type zeolites to relieve diverse pathologies such as stomach acidity (Linares et al. 2005) and hypercholesterolemia (Linares et al. 2008). Cancrinites have a Si/Al ratio equal to 1, which is favourable for the cationic exchange procedures (Buttersack et al. 1993). Therefore, this work represents a new opportunity offered by modified cancrinite zeolites as a glucose adsorbent.
MATERIALS AND METHODS
A nitrate-sodium cancrinite zeolite was synthesized according to the previously reported procedure (Hackbarth et al. 1999). Then, this cancrinite was ion-exchanged with 0.01M Ca2+, Mg2+ or Fe2+ solutions by using a ratio of 10 mL salt solution/g zeolite. The slurry was kept in reflux for 24h, centrifuged and the solid was refluxed again twice. Fe2+ oxidation state was preserved by using a N2 atmosphere during the reflux condition. After the last reflux, the slurry was centrifuged again and solids were washed off with abundant distilled water and dried at 80°C for 18 h in a convection oven. Samples were identified as Na-Can (sodium-cancrinite), Mg-Can (magnesium-cancrinite), Fe-Can (iron (II) cancrinite) and Ca-Can (calcium-cancrinite). Obtained solids were characterized by techniques such as: X-ray diffraction (XRD), Fourier transforms infrared spectroscopy (FT-IR), N2 physisorption measurements and chemical analysis. XRD analyses were performed in a Siemens 5000 difractometer with CuKa radiation (1.542 Å C5 Å ) for crystalline phase detection from 5 to 80° (2Θ). The presence of functional groups and an evaluation of purity of solids were achieved by FT-IR. Spectra were recorded in a Perkin-Elmer 283 spectrometer in the 4000-400 cm-1 range. Physisorption measurements were carried out in a Beckman Coulter SA 3100 instrument; BET surface areas were determined by nitrogen adsorption at -196°C with an Ar/N2 ratio of 70/30. Inductively Coupled Plasma Emission Spectroscopy (ICP) using a Perkin Elmer ICP/ 5500 instrument was used to analyze the chemical compositions of the original and exchanged zeolites.
Characterized cancrinites were contacted with 3mL of a glucose solution (100 ppm) at different masses: 50, 100, 200 and 500 mg for 60 min. In order to determine the contact time, 100 mg of solids were also put in contact with a glucose solution at 15, 30, 45 and 60 min. Solids were centrifuged and analyzed by FT-IR. The quantitative determination of glucose was performed by enzymatic techniques whose coloured complex was followed by UV-Visible at 506 nm.
RESULTS AND DISCUSSION
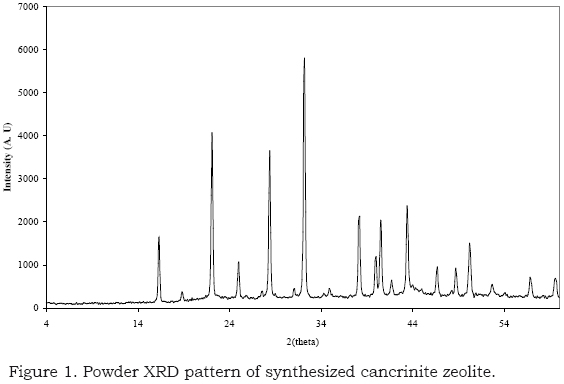
The XRD pattern of the Na+ cancrinite sample shows reflections attributed to the nitrated-cancrinite-type zeolite that was consistent with the P63 spatial group (ICDD-PDF# 38-0513) (Robson and Lillerud 2001) (Fig. 1). Other phases, such as sodalite, were not detected by XRD. XRD patterns for the exchanged cancrinites were similar to that reported by nitrate-sodium cancrinite.
On the other hand, FT-IR spectrum (Fig. 2) of the synthesized sodium cancrinite-type zeolite showed different bands: a band at 1425 cm-1 was assigned to the presence of nitrate anions occluded in the zeolite framework structure. The band placed at 1631 cm-1 corresponds to water molecules localized inside the cancrinite cavity, while bands in the region between 1095 and 500 cm-1 were attributed to Si-O-Al bonds (Hackbarth et al. 1999).

Likewise, Table 1 shows the chemical analysis and textural properties of exchanged cancrinites. Because Mg and Ca are di-valence cations, they are exchanged in minor proportion in comparison to monovalence cations.

The BET specific surface area of Na-Can is quite low if it is compared to other zeolites whose pores are free. The presence of nitrate anions occluded inside the framework structure is responsible for this low surface area (Hackbarth et al. 1999). As the pores are blocking, the BET area is almost entirely the external area of these solids. The micropores area is quite negligible. These results are expanded to the other exchanged cancrinites.
When, the Na-Can is exchanged with Mg2+ or Ca2+ cations, the BET surface area is slightly increased in comparison to Na-Can. Due to the fact that, Mg+2 and Ca2+ cations are di-valences, they allow a major access to the cancrinite pores
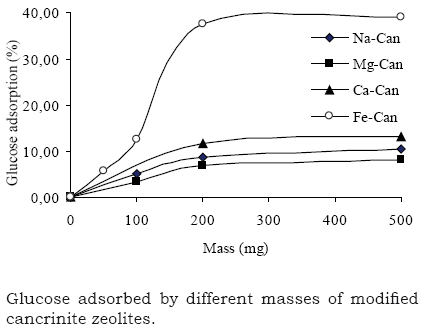
The previously characterized samples were, then, tested as glucose adsorbents. As it is depicted in Fig. 3, the adsorption process is largely enhanced during the first 200 mg sample; after that, the adsorption rate remains constant until the 500-mg sample. A possible explanation could be associated to mass transference troubles. This glucose adsorption takes place on the external crystal surface due to the pore blocking showed by the cancrinite type-zeolites as showed in Table 1 (Hackbarth et al. 1999).

On the other hand, it was observed a preference on the glucose adsorption when Fe-Can (~ 40%) was used in comparison with the other ion-exchanged zeolites (~5-10%). According to literature (Wolowiec and Drabent 1985), Fe2+ can produce a stable complex with glucose molecules while glucose interactions with Ca2+ or Mg2+ zeolites are weaker (Angyal 1984). Mg-cancrinite showed the lowest adsorption capacity among all the tested cations. According to Heper et al (2007), Mg2+-zeolite Y presents lack of free space inside the zeolite pores to accommodate glucose. The reason for this could be a higher affinity of the zeolite to Mg2+ or a greater hydration layer of Mg2+ resulting in its inability to leave the cages.
Previously, Sherman and Chao (1989) and Kulprathipanja (1991), had reported the preference of Ca2+ and K+ cations for fructose or glucose molecules. However, K+ and Na+ are considered non-complex ions, as it has been reported that sugars and univalent cations form very weak complexes in comparison to divalent cations (Churm 1996, Nobre et al. 2009).
Concepción-Rosabal et al . (1997) reported the use of Fe+2, Ca2+ and Mg2+ ion-exchanged clinoptilolites as glucose adsorbent. They found an adsorption order similar to our results: Fe>>Mg>Ca ≈ Na. However, no comparison related to the glucose adsorption capacity could be established because the glucose concentration in the Concepción-Rosabals work was not reported.
Likewise, the time influence on the glucose adsorption was also determined for modified cancrinites using 100 mg of sample (Fig. 4). In general, the adsorption of glucose increased as contact time is increased.

A maximum adsorption was determined at 60 min of contact time. This time is ideal if gastric functions are carried out (Linares et al. 2005). On the other hand, the adsorption behaviour by exchanged cations was similar to that reported for mass influence.
In order to investigate the controlling mechanism of adsorption processes such as mass transfer or chemical reaction, the pseudo-first-order and pseudo-second order equations were applied to model the kinetics of glucose adsorption onto exchanged cancrinites
The possibility of adsorption data to follow Lagergren pseudo-first order kinetics (Lagergren 1898) is given by:
dq/dt = K1 (qe-q) (1)
by integrating Eq (1), the kinetic rate expression becomes:
log(qe- qt) = log qe- (K1 *t)/2.3 (2)
The first order rate constant K1 can be obtained from the slope of plot between log (qe - q) versus time t.
A pseudo-second order model proposed by Ho and Mckay (Ho and McKay 1999) can be used to explain the sorption kinetics. This model is based on the assumption that the adsorption follows second order chemisorptions (Ho 2004). The pseudo-second order can be expressed as:
dq/dt = K11. (qe-q)2 (3)
dq/(qe -q)2 = K11. dt (4)
Integrating Eq 4 simplifies it to:
t/qt = 1/(K11. qe2) + t/qe (5)
where t is the contact time, min, qe and qt are the amount of glucose adsorbed by zeolite (mg/g) at equilibrium and at any time t. A plot between t/qt versus t gives the value of the constant K11 (g(mg.min)-1) and also qe, mg/g, can be calculated.
In this study, the pseudo-first order model fitted better when compared with the pseudo-second order according to r2 and qe values (Table 2). Therefore, the adsorption data in the present study could show a physical adsorption which will depend on the glucose concentration (Kushwaha et al. 2008). A major glucose concentration could increase the adsorption capacity of cancrinites.
CONCLUSIONS
New solids, the ion-exchanged cancrinite-type zeolites, could be used as hypoglycemiant drugs. The best results were obtained with Fe2+ cancrinites while the Ca2+, Mg2+ and Na+ cancrinites displayed much lower adsorption than that of Fe2+. Similarly, the influence of time on the glucose adsorption showed that Fe2+ cancrinite was more active at short contact times than that showed by the ion-exchanged zeolites with other cations.
ACKNOWLEDGEMENT
This work was supported by CDCH-UC. Thanks to Ilse Rodríguez de Georges for checking the manuscript.
REFERENCES
Angyal, S. J. (1984) The composition of reducing sugars in solution. Advances in Carbohydrate Chemical and Biochemistry 42: 15-68. [ Links ]
Buttersack, C., Wach, W., Buchholz, K. (1993) Specific adsorption of saccarides by dealuminated Y-zeolites. Journal of Physical Chemistry B 97: 11861-11862. [ Links ]
Concepción-Rosabal, B., Rodríguez-Fuentes, G., Simón-Carballo, R. (1997) Development and featuring of the zeolitic active principle FZ: a glucose adsorbent. Zeolites 19: 47-50. [ Links ]
Churms, S. C. (1996) Recent progress in carbohydrate separation by high-performance liquid chromatography based on size exclusion. Journal of Chromatography A 720: 151-166. [ Links ]
Hackbarth, K., Fechtelkord, T., Stief F., Buhl, J. (1999) Synthesis and crystal structure of carbonate cancrinite Na8[AlSiO4]6CO3(H2O)3.4, grown under low-temperature hydrothermal conditions. Microporous and Mesoporous Materials 30: 347-358. [ Links ]
Heper, M., Türker, L., Kincal N. S. (2007) Sodium, ammonium, calcium, and magnesium forms of zeolite Y for the adsorption of glucose and fructose from aqueous solutions. Journal of Colloid and Interface Science 306: 11-15. [ Links ]
Ho, Y. S. (2004) Citation review of Lagergren kinetic rate equation on adsorption reaction. Scientometrics 59: 171-177. [ Links ]
Ho, Y.S., McKay, G. (1999) Pseudo-second order model for sorption processes. Process Biochemistry 34: 451-465. [ Links ]
Kulprathipanja S (1991) Pat. 5000794. [ Links ]
Kushwaha, S., Sodaye, S., Padmaja, P. (2008) Equilibrium, kinetics and thermodynamic studies for adsorption of Hg (II) on palm shell powder. World Academy of Science, Engineering and Technology 43: 600-606. [ Links ]
Lagergren, S. (1898) Zur theorie der sogenannten adsorption gelöster stoffe. Kungliga Svenska Vetenskapsakademiens. Handlingar, Band 24: 1-39. [ Links ]
Linares, CF., Colmenares M., Ocanto F., Valbuena, O. (2008) Human bile sorption by cancrinite-type zeolites. Material Science and Engineering C 29: 350-355. [ Links ]
Linares, CF., Sánchez, S., Urbina de Navarro, C., Rodríguez, K., Goldwasser, M. R. (2005) Study of cancrinite-type zeolite as possible antacid agents. Microporous and Mesoporous Materials 77: 215-221. [ Links ]
Nobre, C., Santos M. J., Dominguez, A., Torres, D., Rocha O., Peres A. M., Rocha, I., Ferreira E. C., Teixeira J. A., Rodríguez, L. R. (2009). Comparison of adsorption equilibrium of fructose, glucose and sucrose on potassium gel-macroporous sodium ion-exchange resins. Analytical Chimica Acta 654: 71-75. [ Links ]
Robson, H., Lillerud, KP. (2001) Verified Synthesis of Zeolitic Materials, 2nd ed., ed. by Elsevier, Amsterdam, pp. 27. [ Links ]
Sherman, J. D., Chao, Ch. C.(1989) Pat. 4471114. [ Links ]
White, A., Handler, P., Smith, E. (1959) Principles of Biochemistry, 3er ed, Edt. Mcgraw-Hill, New York (USA) pp. 896-898. [ Links ]
Wolowiec, S., Drabent, K. (1985) Mössbauer study of Fe/III/-reducing sugar complexes, Journal of Radioanalytical and. Nuclear Chemistry 95: 1-2. [ Links ]














