Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista latinoamericana de química
versión impresa ISSN 0370-5943
Rev. latinoam. quím vol.38 no.2 Naucalpan de Juárez ago. 2010
Secondary metabolites and pharmacology of Foeniculum vulgare Mill. Subsp. Piperitum
Mahmoud I. Nassarª*, El–sayed A. Aboutablb, Yousrya A. Makledc, Ezzel–DIN A. El–Khrisyª, Abeer F. Osmanª
ª Chemistry of Natural Compounds Department, National Research Centre, 12622 Dokki, Cairo, Egypt. *Corresponding author: Tel. 20222337651; Fax: 20233370931. Email: mnassar_eg@yahoo.com
b Pharmacognosy Department, Faculty of Pharmacy, Cairo University, Kasr–el–Aini St., 11562 Cairo, Egypt.
c Pharmaceutical and Medicinal Chemistry Department, National Research Centre, 12622 Dokki, Cairo, Egypt.
Received March 2010.
Accepted August 2010.
ABSTRACT
From hexane extract of Foeniculum vulgare Mill. Subsp. piperitum the fatty acids, hydrocarbons and sterols were identified. The furocoumarins imperatorin, psoralen, bergapten, xanthotoxin and isopimpinellin were isolated from the methylene chloride extract. The flavonoids isorhamnetin 3–O–α–rhamnoside, quercetin and kaempferol were isolated from the ethyl acetate extract, whereas quercetin 3–O–rutinoside, kaempferol 3–O–rutinoside and quercetin 3–O– β–glucoside were isolated from the methanol extract. The crude hexane, methylene chloride, ethyl acetate and methanol extracts of this plant showed antinociceptive and anti–inflammatory activity.
Keywords: Foeniculum vulgare Mill. Subsp. piperitum, coumarins, flavonoids, antinociceptive, antiinflammatory.
RESUMEN
Se realizó la investigación de la constitución química y la actividad biológica de Foeniculum vulgare Mill. Subsp. Piperitum. En el extracto de n–hexano de Foeniculum vulgare Mill. Subsp. Piperitum. se identificaron ácidos grasos, hidrocarburos y esteroles. Las furocumarinas imperatorina, psoraleno, bergapteno, xantotoxina y isopimpinelina se aislaron del extracto de cloruro de metileno. Los flavonoides 3–O–α–ramnosido de isoramnetina, quercetina y kaempferol se aislados del extracto de acetato de etilo, en tanto que la 3–O–rutinósido de quercetina, el 3–O–rutinósido de kaempferol y 3–O–β–glucósido de quercetina se aislaron del extracto metanólico. Los extractos crudos de n–hexano, cloruro de metileno,acetato de etilo y metanol presentaron actividad antinociceptiva y anti–inflamatoria.
INTRODUCTION
Foeniculum vulgare Mill. subsp. piperitum (Ucria) Coutinho (Fam. Apiaceae) grows wildly in the Mediterranean coastal strip, Egypt (Tackholm, l974). It has been widely used as a folk remedy by the native people for treatment of various inflammatory ailments. Chemically, Foeniculum species are characterized by the presence of essential oils (Ozbek et al.,2003), sterols (Ivanov et al., 1979), coumarins (El–Khrisy et al., 1980; Kwon, et al., 2002) and flavonoids (Kunzemann et al., 1977; Parejo et al., 2004). Certain bioactivities have been attributed to some Foeniculum species; viz, antioxidant and antimicrobial activities for F. vulgare Mill. aerial parts (Ruberto et al., 2000), anti–inflammatory and analgesic activities for the fruits of the same plant ( Eun and Jae, 2004). Volatiles reported from the fruits of F. vulgare subsp. piperitum comprise anethol, methyl chavicol, fenchone and limonene (Muckensturm et al., 1997), as well as piperitenone and piperitenone oxide (Badoc et al., 1994). Chlorogenic acid (Ishikawa et al., 1999), caffeic acid and cynarin (Scarpati, 1957) have also been isolated from the plant. The purpose of this study was the isolation and identification of chemical constituents from Foeniculum vulgare Mill. subsp. piperitum growing in Egypt, as well as the evaluation of antino–ciceptive and antiinflammatory activities of crude extracts of the plant.
PLANT MATERIAL
The aerial parts and fruits of Foeniculum vulgare Mill. subsp. piperitum were collected from North Western Mediterranean coastal strip near El–Salloum, Egypt in October 2005. The plant material was identified by Prof. Dr. S.A. Kawashty, Phytochemistry and Plant Systematics Department, NRC, Cairo, Egypt. Voucher specimen (F121) is kept in Herbarium, Pharmacognosy Department Faculty of Pharmacy, Cairo University.
EXTRACTION AND ISOLATION
Successive extracts of the air–dried powdered aerial parts (1.8 kg) and fruits (150 g) of Foeniculum vulgare Mill. subsp. piperitum were prepared using n–hexane, methylene chloride, ethyl acetate and methanol, in succession. n–Hexane extracts (7 g) from each of the aerial parts and fruits were saponified (El–Said and Amer,1965) to yield the unsaponifiable matter fractions (USM) (3.5 g and 4 g, respectively), and fatty acids fractions (FA) (2.0 g and 2.5 g, respectively). Methylation of FA was carried out by refluxing in 50 ml absolute methanol and 1.5 ml sulphuric acid for 2 h to give fatty acids methyl esters.
GLC of unsaponfiable matter (USM)
Agilent 6890N gas chromatograph (Hewlett Packard) equipped with FID (Flame Ionization Detector) was used. Column: capillary, 30 m length, 0.53 mm internal diameter, film thickness 0.5 pm, packed with 5% phenyl – 95% dimethyl polysiloxane; temperature program: 80ºC, for 1min, increased at a rate of 8ºC/min.; final temperature, 250ºC (kept for 20 min). Injector temperature: 280ºC; detector temperature: 300ºC; carrier gas: nitrogen, flow–rate 30 ml/min; H2 flow–rate: 30 ml/min.; air flow–rate, 300ml/min.
GLC of fatty acids methyl esters (FAME)
Agilent 6890N gas chromatograph (Hewlett Packard) equipped with FID was used. Column: capillary, 30 m length, HP–INWAX Polyethylene glycol, 320 pm internal diameter, 0.25 μm film thickness; temperature program: 70ºC for 2min., increased at a rate of 4ºC/min. till 220ºC; carrier gas: nitrogen, flow–rate, 30 ml/min.; air flowrate, 230 ml/min.
Isolation of coumarins from methylene chloride extract:
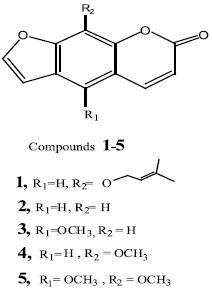
The solvent–free methylene chloride extract (greenish brown, 37g) was subjected to silica gel CC (75 x 3cm, 150 g) and eluted with benzene and step–gradient benzene/ethyl acetate; 50 ml fractions being collected, monitored by TLC using solvent system benzene/ethyl acetate (8:2) and similar fractions were pooled. Fractions eluted with benzene afforded compound 1 (20 mg), while those eluted with benzene/ethyl acetate (9.5:0.5) afforded compound 2 (18 mg), Fractions eluted with benzene/ethyl acetate (9:1) gave a mixture (1.5 g) of compounds 3,4 and 5 which was subjected to silica gel CC (30 x 2 cm, 25g), eluted successively with benzene, benzene/ethyl acetate (9.5: 0.5) and benzene/ethyl acetate (9:1); yielding 60 subfractions (each, 20 ml). Subfractions eluted with benzene/ethyl acetate (9.5:0.5) afforded compounds 3 (20 mg), and 4 (22 mg), while those eluted with benzene/ ethyl acetate (9:1) afforded compound 5 (15 mg).
Isolation of flavonoids from ethyl acetate extract
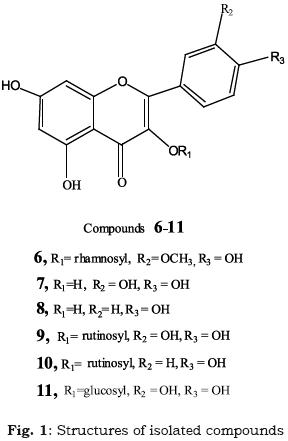
The ethyl acetate extract (24 g) was subjected to polyamide 6S CC (100 x 4 cm, 200 g). Elution with water, followed by methanol (40 %, 60 %, 80 % and 100 %, in succession) yielded 130 fractions (200 ml, each) which were monitored by PC using BAW and 15 % AcOH as developing systems; similar fractions being pooled. Fractions eluted with 80%methanol yielded the isolate 6 (23 mg) which was further purified by Sephadex LH–20 CC (30 × 2cm, 40g) using methanol as eluent. Fractions eluted with methanol afforded a mixture of two compounds which were separated and purified by Sephadex LH–20 CC (30 × 2cm, 40g) using methanol as eluent, to give the compounds 7 (30 mg) and 8 (28 mg).
Isolation of flavonoids from the methanolic extract
The methanolic extract (77 g) was treated as the ethyl acetate extract. The fractions eluted with 40 % MeOH yielded the isolate 9 (22 mg), those eluted with 60% MeOH afforded the isolate 10 (30 mg), while fractions eluted with 80% MeOH yielded the isolate 11 (20 mg). Each isolate was further purified by Sephadex LH–20 CC, using methanol as eluent.
Evaluation of Bioactivities
The powdered air–dried aerial parts of F. volgare Sups. piperitum (1.2 kg) was successively extracted with n–hexane, methylene chloride, ethyl acetate and methanol to yield 40, 25, 16 and 70 g of extractives, respectively. 1 mg of the individual extracts was equivalent to 30, 48, 75 and 17.1 mg dry plant, respectively. The extracts were suspended in 7% Tween 80 and biologically tested in different doses. All doses were expressed in terms of extract weight/animal body weight (Berhrens and Kerber, 1953).
Animals and Reference drugs
Adult male albino rats weighing (120–150 g) and adult albino mice of both sex (2025 g) used in this study, were obtained from the animal–breeding unit of National Research Centre, Cairo. The animals were fed a standard laboratory diet composed of vitamin mix (1 %), mineral mix. (4 %), corn oil (10 %), sucrose (20 %), cellulose (0.2 %), casein (10.5 %) and starch (54.3 %) and allowed free access to water. Animal procedures were in accordance with the recommendations for the proper care and use of laboratory animals. This study was performed according to the international rules and to the guidelines of ethical comity of National Research Centre for experimental animal use. Acetylsalicylic acid (El Nasr Co., Egypt), Ibuprofen (Egyptian International Pharma. Industries Co., Egypt), Carrageenan (BDH, England) and Fluconazole (Pfizer INC., USA) were used in bioactivity testing.
Acute toxicity (LD50)
Acute toxicity (LD50) of each of the extracts from the plant was determined in mice (Finney, 1964). They were subcutaneously (S.C.) administered in doses ranging from 4 to 13 g/kg body weight. Animals were observed and mortality rates were recorded within the first 24 h after administration. Different extracts assayed up to 5.5 g/kg did not show toxicity. LD50 being: 6.75, 11.0, 6.92 and 15 g/kg for n–hexane, methylene chloride, ethyl acetate and methanol extracts, respectively.
Antinociceptive activity
This activity was investigated using the writhing method in mice (Margarita et al., 1995). The tested extracts were administered in doses ranging 200–2000 mg/kg as well as acetylsalicylic acid (200 mg/kg) as a peripheral antinociceptive reference drug (Margarita et al., 1995) and the control vehicle (7% v/v between 80 in normal saline) were S.C. injected to groups of six animals 15 minutes before the intraperitoneal injection of a freshly prepared acetic acid solution (2% w/v in saline, pH 2.7, 10 ml/kg) and transferred immediately to individual observation cages and the assay was observed over a period of 25 min. The number of abdominal writhes was counted and the percentage of protection was expressed as the following:
 Where: C = mean writhing of the control group; T = mean writhing of the treated group
Where: C = mean writhing of the control group; T = mean writhing of the treated group
Anti–inflammatory activity
Anti–inflammatory activity was studied using Carrageenan–induced rat's paw edema (Winter et al., 1963). Groups of 18 h–fasted male rats (110–130 g, 6 animals each) were orally–dosed with either one of the tested extracts from Foeniculum vulgare 1h before induction of Carra–geenan foot paw edema by subplanter injection of 0.05 ml of 1% suspension of Carrageenan in saline into the planter tissue of one hind paw. An equal volume of saline was injected into the other hind paw and served as control. Four hours later, the animals were sacrificed and the hind paws were rapidly amputated at the tibiotarsal joint and weighed (Margarita et al., 1995; El–Azzouny et al., 1995). The average weight of edema was estimated for the treated as well as the control group and the percentage inhibition of weight of edema was also evaluated. Ibuprofen (35 mg/kg) (Makhlouf and Maklad, 2004) was employed as a standard.
IDENTIFICATION: SPECTRAL DATA
GLC analysis of FAME (Table 1) revealed 15 compounds in the aerial parts and 14 compounds in the fruits. In the aerial parts and fruits, saturated fatty acids represent 68.43 and 75.03%, respectively; the major being: palmitic, undecanoic and myristic acids, while the unsaturated acids represent 22.01% and 20.32%, respectively; the major being pentadecadienoic and pen–tadecenoic acids. GLC of USM revealed 27 compounds in the aerial parts, as well as the fruits (Table 2). Hydrocarbons (56.51, 54.17%),, cholesterol (1.71, 0.84%), β–sitosterol (5.52, 5.95%), campesterol (3.33, 4.04%) and stigmasterol (14.86, 19.04%) were identified in the aerial parts as well as the fruits, respectively.
Imperatorin (1): UV λmax (MeOH): 262 and 300 nm. EI–MS: m/z 270 [M+, base peak, C16H14O4]; m/z 202 [M+ –C5H9 ]; m/z 174 [M+ –C5H9– CO]; m/z 146 [M+ –C5H9–CO–CO]; 1H–NMR (CDCl3, 270 MHz): δ 7.76 (H–4, d, J = 9.5 Hz), δ 7.73, (H–5,s), δ 7.34 (H–7, d, J = 2.5 Hz), δ 6.79 (H–6,d, J = 2.5 Hz), δ6.35 (H–3, d, J = 9.5 Hz), δ5.58 (H, t, J = 6.5 Hz), δ4.99 (two protons for OCH2, d, J = 7.1 Hz), δ1.69 and δ1.71 (singlets for the two methyls).
Psoralen (2): UV λmax (MeOH): 246 and 293 nm. EI–MS: m/z 186 [M+, base peak, CHOI which loses three carbon monoxide molecules, successively, to give the fragments m/z: 158, 130 and 102. 1H–NMR (CDCl3, 270 MHz): δ7.48 (H–4, d, J = 9.5 Hz), δ7.36 (H–5, s), δ7.35 (H–9, s), δ7.13 (H–7,d, J = 2.5 Hz), δ6.50 (H–6 ,d, J = 2.5), δ 6.06 (H–3,d, J = 9.5 Hz).
Bergapten (3): UV λmax (MeOH): 249, 259, 267 and 310 nm. EI–MS: m/z 216 [M+, C12H8O3], m/z 201 [M+–CH3 ], followed by four successive expulsions of carbon monoxide to give the fragments m/z: 173, 145, 117 and 89. 1H–NMR (CDCl3, 270 MHz): δ8.13 (H–4, d, J = 9.7 Hz), δ7.57 (H–7, d, J = 2.5Hz), δ7.08 (H–9, s), δ7.00 (H–6, d, J = 2.5 Hz), δ 6.25 (H–3, d, J = 9.7 Hz), δ 4.25 (singlet, 5–OCH3).
Xanthotoxin (4): UV λmax (MeOH):240 and 298 nm. EI–MS: m/z 216 [M+, C12H8O3], m/z 201 [M+–CH3], followed by four successive expulsions of carbon monoxide to give the fragments: m/z 173, 145, 117 and 89. 1H–NMR (CDCl3, 270 MHz): δ 7.76 (H–4, d, J = 9.5 Hz), δ 7.67 (H–7, d, J = 2.1 Hz), δ 7.32 (H–5, s), δ6.80 (H–6, d, J = 2.1 Hz), δ6.35 (H–3, d, J = 9.5 Hz), δ 4.26 (singlet, 9–OCH3).
Isopimpinellin (5): UV λmax (MeOH): 248, 267and 309 nm. EI–MS: m/z 246 [M+, C13H10O5], m/z 231 [M+–CH3], followed by two successive expulsions of carbon monoxide to give the fragments m/z 203 and 175, and loss of methyl group to give the fragment m/z 160. 1H–NMR (CDCl3, 300 MHz): δ8.14 (H–4, d, J = 9.5 Hz), δ7.64 (H–7, d, J = 2.5 Hz), δ7.06 (H–6, d, J = 2.5 Hz), δ6.36 (H–3, d, J = 9.5 Hz) and two singlets at δ 4.13 and 4.09 for 5– and 9–OCH3.
Isorhamnetin 3–O–α–L–rhamnoside (6):
EI–MS: m/z 316 [Aglycone+H]+, +ve ESI–MS: m/z 462 [M+H+, C22H22O11]. 1H–NMR (DMSO–d6, 270 MHz): δ12.63 (5–OH,s), δ7.27 (H–2', d, meta–coupling with H–6', J = 2.0Hz), δ7.25 (H–6' d, J = 8.8 Hz), δ 6.85 (H–5\ d, J = 8.3 Hz), δ 6.32 (H–8, d, J = 2.0 Hz), δ 6.13 (H–6, d, J = 2.0 Hz ), δ5.24 (broad singlet for anomeric proton of rhamnose), δ0.95 (CH3 methyl rhamnosyl protons, d, J = 5.3 Hz), δ3.95 (OCH3 at position 3', sharp singlet) (Chang et al., 1998).
Quercetin (7): UV spectral data suggested a flavonol type with free hydroxyl groups at positions 3, 5, 7, 3' and 4'. EI–MS: m/z 303 [M+1]+, C15H10O7.
Kaempferol (8): UV spectral data suggested a flavonol type with free hydroxyl groups at positions 3, 5, 7 and 4'. EI–MS: m/z 285 [M–1] –, C15H10O6.
Quercetin 3–O– rutinoside (9): 1H–NMR: δ7.55 (H–2' and H–6' overlapping protons, d, J = 7.6 Hz), δ 6.85 (H–5', d, J = 8.0 Hz), δ 6.38 (H–8 , d, J = 1.8 Hz), δ 6.19 (H–6, d, J = 1.8 Hz), ,δ5.32 (anomeric proton of glucose, J = 8.1 Hz), δ 4.37 (anomeric proton of rhamnose, broad singlet), δ0.99 (CH3–rhamnosyl, d, J = 5.4 Hz).
Kaempferol 3–O– rutinoside (10): UV spectra with shift reagents indicated a flavonol type with free hydroxyl groups at positions 5, 7 and 4', while that at position 3 being substituted (Mabry et al., 1970). Complete acid hydrolysis afforded glucose, rhamnose and kaempferol, identified by CoPC in comparison with authentic samples. 1H–NMR (DMSO–d6, 270 MHz): δ7.61 (H–2'and H–6', d, J = 8.5 Hz) δ 6.77 (H–3' and H–5', d, J = 8.5 Hz) , δ6.20 (H–8 , d, J = 1.8 Hz) , δ6.0 (H–6, d, J = 1.8 Hz), δ 5.21 (anomeric proton of glucose, d, J = 7.6 Hz), δ 4.40 (anomeric proton of rhamnose, broad singlet) δ 1.04 (methyl rhamnosyl protons, d, J = 6.1 Hz). ESI–MS: m/z 593 [M–1]+, C21H20O12.
Quercetin 3–O–β – glucoside (11): UV spectral data suggested a flavonol type with free hydroxyl groups at positions 5, 7, 3' and 4', while being substituted at position 3. Complete acid hydrolysis afforded glucose. 1H–NMR: δ 7.66 (H–6', d, J = 8.6 Hz), δ 7.55 (H–2', d, J =2.0 Hz) , δ 6.84 (H–5', d, J = 6.6 Hz), δ 6.38 (H–8, d, J = 1.8 Hz), δ 6.17 (H–6, d, J = 1.8 Hz), δ5.34 (anomeric proton of glucose, d, J = 7.5 Hz). ESI–MS: m/z 464 [M+1] +, C21H20O12.
Bioactivities
The methanolic extract of the aerial parts of Foeniculum vulgare subsp. piperitum exhibited the highest antinociceptive activity at a dose level of 2000 mg/kg (Table 3), while the activity exhibited by the ethyl acetate extract was at (800 mg/kg). On the other hand, n–hexane extract (700 mg/kg) and methylene chloride extract (500 mg/kg) exhibited similar antinociceptive activities, being less than that of acetylsalicylic acid (200 mg/kg). Data presented in Table 4 revealed that the extracts under investigation exhibited significant anti–inflammatory activity. The methanolic extract possessed the highest activity, where it significantly decreased the weight of edema induced by carrageenan in the rat paw at dose levels of 1500 and 2000 mg/kg, it exerted a protective effect of 28 and 47%, respectively, compared to the control value, while ibu–profen (35 mg/kg), used as a reference drug, exhibited a protective effect of 52.23 %. The present study showed that the four extracts of the aerial parts of the plant exhibited antinociceptive activity. Moreover, the potent antinociceptive activity expressed by the methanolic (2000 mg/kg) and ethyl acetate (800 mg/kg) extracts can be attributed to the content of phenolic constituents (Paulino et al., 2003). Also, the four extracts under investigation exhibited a significant anti–inflammatory activity. The highest anti–inflammatory activity of methanolic extract (2000 mg/kg) of the aerial parts of the plant can possibly be attributed to the presence of phenolic content. In conclusion, traditional application of F. vulgare Mill subsp. piperitum could be beneficial in the management of different inflammatory disease cases. Our current findings suggested that F. vulgare Mill subsp. piperitum contains a high amount of flavonoids, which exhibited a strong potency towards suppressing inflammation, as evidenced by our in vivo models.
CONCLUSION
Foeniculum vulgare Mill. Subsp. Piperi–tum to contain fatty acids, hydrocarbons and sterols in the n–hexane extract. Fu–rocoumarins; imperatorin, psoralen, bergapten, xanthotoxin and isopimpinellin were isolated from the methylene chloride extract. Flavonoids; isorhamnetin 3–O–α–rhamnoside, quercetin and kaempferol were isolated from the ethyl acetate extract, whereas quercetin 3–O–rutinoside, kaempferol 3–O–rutinoside and quercetin 3–O– β–glucoside were isolated from the methanol extract. The crude extracts exhibited significant antinociceptive and anti–inflammatory activity.
REFERENCES
Abdel–Hay, F., Abu–Mustafa, E. A., Fayez, M. B. (1966) Isolation of isopimpinellin from Ammi majus L. natural coumarins.IV. Naturwissenschaften 53: 406–407. [ Links ]
Badoc, A., Deffieux, G., Lamarti, A., Bourgeois, G., Carde, J. P. (1994) Essential oil of Foeniculum vulgare Mill. (fennel) subsp. piperitum (Ucria) Cout. fruit. Journal Essential Oil Research 6: 333–336. [ Links ]
Behrens, B., Kerber, J. (1953) Wie sind reichenversuche fur biologische auswertungen am zweckmassigsten anzwerdnen. Archive Ex. Path Pharm 177: 379–388. [ Links ]
Cuendet, M., Potterat, O., Hostettmann, K. (2001) Flavonoids and phenylpropanoid derivatives from Campanula barbata, Phytochemistry 56: 1631–1636. [ Links ]
El–Azzouny, A. A., El–Shabrawy, O. A., El–Azzouny, M. M., Ebeid, M. Y., Lehmann, J. (1995) Synthesis and pharmacological evaluation of fenamate analogues: 2–[(7–Substuted–4–quinolinyl)–amino] benzoic acid esters. Scientia Pharmacentica 63: 81–92. [ Links ]
El–Khrisy, E. A. M., Mahmoud, A. M., Abu–Mustafa, E. A. (1980) Chemical constituents of Foeniculum vulgare fruits, Fitoterapia 51 : 273 –275. [ Links ]
El–Khrisy, E. A. M., Meshaal, S. A., Abdel–Hafez, O. M., Khattab, A. A., Abu–Mustafa, E. A. (1985) The constituents of Ficus eriobryoides, Ficus cunnghamii and Ficus sycomorus leaves. Fitoterapia 56: 184–187. [ Links ]
El–Said, M. E., Amer, M. M. (1965) Oils, fats, waxes and surfactants. Anglo–Egyptian Bookshop, Cairo, Egypt. [ Links ]
Eun, M. C., Jae, K. H. (2004) Anti–inflammatory, analgesic and antioxidant activities of the fruit of Foeniculum Vulgare. Fitoterapia 75: 557–565. [ Links ]
Finney, D. J. (1964) Statistical methods in biological assay. Charles Griffin Company limited, London. [ Links ]
Ishikawa, T., Tanaka, Y., Kitajima, J., Ida, Y. (1999) Water–soluble constituents of fennel. viii. monoterpenoid alcohols and thujane–, camphene–, norfenchane–type monoterpenoid glycosides. Chemical Pharm Bulletin 47: 805–808. [ Links ]
Ivanov, S., Seher, A., Schiller, H. (1979) Natural antioxdants. IV: Antioxidants in the fatty oil of Foeniculum vulgare Mill. 2. Fette, Seifen, Anstrichm. 81: 105–107. [ Links ]
Kunzemann, J., Herrmann, K. (1977) Isolation and identification of flavonol–O–glycosides in Caraway (Carum Carvi), fennel (Foeniculum vulgare), anise (Pimpinella anisum) and Coriander (Coriandrum sativum) and of flavone–C–glycosides in anise. I. Phenolics of spices. Untersuchung Zeitschrift fur Lebensmittel Forschung 164: 194–200. [ Links ]
Kwon, Y. S., Choi, W. G., Kim, W. J., Kim, W. K., Kim, M. J., Kang, W. H., Kim, C. M. (2002) Antimicrobial constituents of Foeniculum vulgare. Archives of Pharmacol Research 25: 154–157. [ Links ]
Mabry, T. J., Markham, K. R., Thomas, M. B. (1970) The Systematic Identification of Flavo–noids. Springer Verlag, New York. [ Links ]
Makhlouf, A. A., Maklad, Y. A. (2004) Synthesis and analgesic–anti–inflammatory activities of some 1, 2, 4–triazine derivatives. Arzneimit Forsch/ Drug Research 54: 42–49. [ Links ]
Margarita, H. P., Rosa, M. R., Carmen dela Torre, M., Rodriguez, B. (1995) Analgesic, anti–inflammatory and haematological effects of aethiopinone and o–naphthoquinone diterpenoid from Salvia aethiopis roots and two hemisynthetic derivatives. Planta Medica 61 : 505–507. [ Links ]
Muckensturm, B., Foechterlen, D., Reduron, J. P., Danton, P., Hildenbrand, M. (1997) Phy–tochemical and chemotaxonomic studies of Foeniculum vulgare. Biochemical Systematics and Ecology 25: 353–358. [ Links ]
Özbek, H., Ugras, S., Düglar, H., Bayram, T., Özturk, G., Özturk, A. (2003) Hepatoprotective effect of Foeniculum vulgare essential oil, Fitoterapia 74: 317–319. [ Links ]
Parejo, I., Viladomat, F., Bastida, J., Codina, C. (2004) Development and validation of a high–performance liquid chromatographic method for the analysis of antioxidative phenolic compounds in fennel using a narrow pore reversed phase C18 column, Anal. Chem. Acta 519: 271–280. [ Links ]
Paulino, N., Dantas, A. P., Bankova, V., Longhi, D. T., Scremin, A., Caixto, J. B. (2003) Journal Pharmaceutical Sciences 93: 307–313. [ Links ]
Ruberto, G., Baratta, M. T., Deans, S. G., Dorman, H. J. D. (2000) Antioxidant and antimicrobial activity of Foeniculum vulgare and Crithmum maritimum essential oils, Planta Medica 66: 687– 693. [ Links ]
Scarpati, M. L. (1957) Oriented search for cynarin in plants containing other caffeyl derivatives, Ann. Chim. 47: 155–162. [ Links ]
Stanley, W. L., Vannier, S.H. (1967) Psoralens and substituted coumarins from oil of lime, Phytochemistry 6: 585–588. Tackholm, V. (1974) Students' Flora of Egypt 2nd.ed., Cairo Univ., Beirut, Lebanon. [ Links ]
Winter, C. A., Risley, E. A., Nuss, G. W. (1963) Anti–inflammatory and antipyretic activities of indomethacin, 1–(p–chlorobenzoyl)–5–methoxy–2–methyl–indoie–3–acetic acid, Pharm Experimental Therapy 141 : 369 –376. [ Links ]
Wu, C. M., Koehler, P. E., Ayres, J. C. (1972) Isolation and identification of xanthotoxin (8–me–thoxypsoralen) and bergapten (5–methoxypsoralen) from celery infected with Sclerotiorum, Appied Microbiology 23: 852–856. [ Links ]














