Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista latinoamericana de química
versión impresa ISSN 0370-5943
Rev. latinoam. quím vol.38 no.1 Naucalpan de Juárez abr. 2010
Acyclic diterpenoid from the red alga Gracilaria foliifera
Walied, M. Alarif a*, Seif–Eldin,. N. Ayyadb, Sultan, S. Al–lihaibib
a Department of Marine Chemistry, Faculty of Marine Sciences, King Abdulaziz University, PO. Box 80207, Jeddah 21589, Saudi Arabia. Tel (work): +966–2–6952383, (mob.): +966–56–0352034, Fax: +966–2–6401747. E–mail address: walied1737@yahoo.com
b Department of Chemistry, Faculty of Science, King Abdulaziz University, PO. Box 80203, Jeddah 21589, Saudi Arabia.
Received January 2010.
Accepted April 2010.
ABSTRACT
The dichloromethane/methanol extract from the red alga Gracilaria foliifera was subjected to a column chromatography which afforded a new (naturally) acyclic diterpenoid 3,7,11,15 tetramethyl–3–hexadec–en–1–ol (1) in addition to two C29 ethylidene steroids fucosterol, (24E)–Stigmasta–5,24(28)–diene–3β–ol (2) and isofu–costerol, (24Z)–Stigmasta–5,24(28)–diene–3β–ol (3) which are reported for the first time from the genus Gracilaria. The structures were assigned mainly on the basis of 1H and 13C NMR experiments.
Keywords: Red Sea, Gracilaria foliifera, Acyclic diterpenoid, Fucosterol, Isofucosterol.
RESUMEN
Del extracto de diclorometano/methanol de la alga roja Gracilaria foliifera se obtuvo por cromatografía en columna un nuevo diterpenoide aciclico (natural) el 3,7,11,15 tetrametiyl–3–hexadec–en–1–ol (1) y también dos esteroides C29 fucosterol etilideno: (24E)–estigmasta–5,24(28)–diene–3β–ol (2) y el isofucosterol, (24Z)–estigmasta–5,24(28)–diene–3β–ol (3). Es el primer reporte de estos compuestos en el género Gracilaria. Las estructuras de los compuestos fueron asignadas a través de las técnicas 1H y 13C RMN.
Keywords: Gracilaria foliifera, diterpenoide aciclico, Fucosterol, Isofucosterol.
INTRODUCTION
Gracilaria foliifera (Gracilariales, Rhodophyta) red alga is probably confined to the Red, Arabian and Indian Seas (Guiry & Freamhainn, 2009). Red algae of the genus Gracilaria are known for the production of agar (Rodriguez et al., 2009), polysaccharides (Chattopadhyay et al., 2008) toxins (Yotsu–Yamashita et al., 2007), Lipids (Hwang et al., 2007) and steroids (Govindan et al., 1993; Das, Srinivas, 1992a; Das, Srinivas, 1992b). Investigation of the dichloromethane/methanol extract of the red alga Gracilaria foliifera has resulted in the isolation of a new acyclic diterpenoid 3,7,11,15 tetramethyl–3–hexadec–en–1–ol (1), (compound 1 was synthesized by Kulkarni et al., 1988) in addition to two C29 ethylidene steroids fucosterol, (24E)–Stigmasta–5,24(28)–diene–3β–ol (2) and isofucosterol, (24Z)–Stigmasta–5,24(28)–diene–3β–ol (3) which are reported for the first time from the genus Gracilaria.
EXPERIMENTAL
Apparatus and materials
Column chromatography was carried out using silica gel (60G Merck). Thin layer chromatography was carried out on aluminum– backed silica gel (F254 Fluka). EI/MS analyses were carried out on a Shimadzu–QP 2010, NMR measurements were performed for solutions in CDCl3, the 13C and 1H NMR data were assigned on the basis of 1H–1H COSY (correlated spec–troscopy), 13C–1H HMQC (heteronuclear multiple quantum coherence spectroscopy) and 13C–1H HMBC (heteronuclear multiple bond correlation spectroscopy) measured on BRUKER 600MHz NMR spectrometer.
Chemical shifts were recorded as δ values in part per million (ppm) relative to tetra–methylsilane. All J values are given in Hz.
Extraction and isolation
Gracilaria foliifera was collected in Nov. 2007, at the southern coast of the Red Sea of Saudi Arabia near Yemen. After air–drying, samples (300g) were ground and extracted with Dichlromethane/methanol (v/v). The extract was concentrated under reduced pressure to yield 7. 6 g of crude material, which was then subjected to chro–matography over a silica gel column using a gradient of n–hexane–ethyl acetate as gradient solvent. Purification of compounds was monitored by sulfuric acid/ethanol as a spray reagent.
Compound 1
The fraction eluted with n–hexane/EtOAc ,9:1, was further purified by preparative TLC on silica gel using n–hexane/EtOAc (7:3) to give compound 1 (60mg, 0.02%) as a colorless oil Rf 0.71 (n–hexane/EtOAc ,32:6); [α]D= +0.18° (c= 1.2, CHCl3). IR (CHCl3) cm–1:3450, 2955, 2920 and 1660. EI–MSm/z: 296 [m, C20H40O]+, 278 [M– H2O]+,263 [M–CH3–H2O]+,71 [C5H11]+, 57 [C4H9]+. 1H NMR and 13CNMR (CDCl3), δ (ppm): (table 1).
Compounds 2 and 3
The fraction eluted with a mixture of n–hex–ane/EtOAc (8:2) is further purified by repeated column chromatography and PTLC on silica gel using n–hexane/EtOAc (7:1) led to the isolation of compounds 2 and 3 as a mixture (80mg) as a white solid of nearly equal Rf = 0. 45 (n–hexane/EtOAc , 32:6) but with slightly different color response up on spraying with sulfuric acid/ethanol and heating at 110°C for few minutes. Running out 2D TLC several times we could separate them into pure forms.
Compound 2 (major): white solid m.p. 121–123°C.EI–MSm/z: 412 [M, C29H48O]+, 397 [M– CH3]+, 379 [M–CH3–H2O]+, 314 (100) [M–C7H14]+, 299 [M–C7H14–CH3]+, 296 [M–C7H14–H2O]+1, 281 [M–C7H14–H2O–CH3]+1, 281 [M–side chain + 2H]+1. H NMR (CDCl3),δ (ppm): 0.86–0.88 (d, J=6.6 Hz, 6H, 26–CH3 and 27–CH3 ), 0.91 (d, J=6.6 Hz, 3H,21–CH3), 0.68 (s, 3H,18–CH3), 1.00 (s, 3H,19–CH3), 1.58 (d, J=6.6 Hz 3H,29–CH3), 3.53 (m,H,H–3), 5.35 (m,H,H–6), 5.18 (q,H,H–28), 2.32 (sep, J= 6.6 Hz, H–25).13C NMR (CDCl3), δ (ppm): (39.75, C–1), (35.20, C–2), (71.80, C–3), (42.33, C–4), (140.74, C–5), (121.70, C–6), (36.14, C–7), (35.78, C–8), (50.10, C–9) , (39.72, C–10), (28.23, C–11), (42.28, C–12), (42.3, C–13), (56.74, C–14), (31.64, C–15), (34.78, C–16), (56.72, C–17), (11.85, C–18), (19.40, c–19), (39.50, c–20), (24.32, c–21), (37.23, C–22), (31.90, C–23), (146.60, C–24), (36.50, C–25), (22.23, C–26), (22.50, C–27), (115.54, C–28), (18.70, C–29).
Compound 3 (minor): white solid m.p. 133–135°C.EI–MSm/z: 412 [M, C29H48O]+, 397 [M– CH3]+, 379 [M–CH3–H2O]+, 314 (100) [M–C6H10O]+.1H NMR (CDCl3),δ (ppm): 0.860.88 (d, J=6.6 Hz, 6H, 26–CH3 and 27–CH3 ), 0.91 (d, J=6.6 Hz, 3H,21–CH3), 0.68 (s, 3H,18–CH3), 1.00 (s, 3H,19–CH3), 1.59 (d, J=6.6 Hz 3H,29–CH3), 3.53 (m,H,H–3), 5.35 (m,H,H–6), 5.11 (q,H,H–28), 2.22 (sep, J= 6.6 Hz, H,H–25).13C NMR (CDCl3), δ (ppm): ( 39.75, C–1), (35.20, C–2), (71.80, C–3), (42.33, C–4), (140.74, C–5), (121.70, C–6), (36.14, C–7), (35.78, C–8), (50.10, C–9), (39.72, C–10), (28.23, C–11), (42.28, C–12), (42.3, C–13), (56.74, C–14), (31.64, C–15), (34.78, C–16), (56.72, C–17), (11.85, C–18), (19.40, c–19), (39.50, c–20), (24.32, c–21), (37.23, C–22), (31.90, C–23), (146.00, C–24), (33.90, C–25), (22.50, C–26), (22.23, C–27), (115.94, C–28), (18.90, C–29).
RESULTS AND DISCUSSION
Compound 1
Compound 1 was obtained as colorless oil and showed a molecular ion peak in EI–MS at m/z 296 which together with 13C NMR data, suggested a molecular formula of C20H40O which indicates 1 degree of unsaturation that can be deduced to be a double bond by examining IR (v 1660 cm–1) absorption spectrum, 1H NMR one olefinic proton at δ 5.41 ppm and 13CNMR two signals at δ 123.02 and 140.38 ppm. The DEPT spectra showed that compound 1 contains five methyl, ten methylene, four methine and one quaternary carbons. The nature of oxygen atom was found to be primary alcoholic from both 1H NMR δ 3.53 (t, J= 6.5) ppm and 13CNMR δ 59.44 ppm. So compound 1 should be acyclic diterpene alcohol which can be deduced to be phytol (4) but comparing our present spectral data with that of phytol (Bang et al., 2002) (table 1) we can observe a difference in the position of the double bond. Localization of the hydroxyl group at C–1, methyl groups and the double bond at C3–C4 were deduced from HMQC, HMBC correlations and COSY experiment. Positions of the methyl groups also can be explained by the obtained spectral data (table 1) which is in agreement with the biogenetic rule of terpenoids. These data of compound 1 allowed to be assigned the following structure, 3,7,11,15 tetramethyl–3–hexadec–en–1–ol.
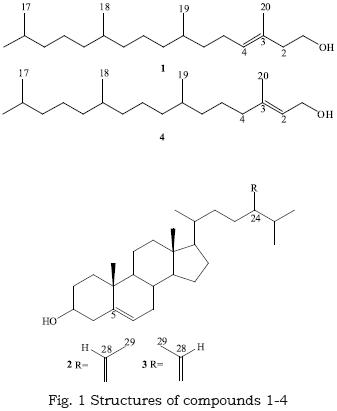
Compounds 2 and 3
GC/MS of the purified steroidal fraction (Liebermann–burchard's reaction gave the typical slow reacting green color of a Δ5 sterol) (Gibbons et al., 1968) gave two closely peaks of nearly equal retention times but with identical MS fragmentation pattern of a parent ion peak at m/z 412 which together with 13C NMR data, suggested a molecular formula of C29H48O. The loss of part of the side chain (C7H14) is characteristic of sterols with a Δ24(28) or Δ24(24') (Gibbons et al., 1968). 1H NMR data were typical of sterols, compound 2 gave a doublet at δ 1.59 ppm (J= 6.6 Hz) for the proton at C–29 (δC 18.70), a quartet δ 5.18 ppm (J= 6.6 Hz) at C–28 proton (δC 115.54), a multiplet at 5.35 for C–6 proton (δC 121.70) and a multiplet at 3.53 for C–3 proton (δC 71.80), while compound 3 gave a doublet at δ 1.58 ppm (J= 6.6 Hz) for the proton at C–29 (δC 18.90), a quartet δ 5.11 ppm (J= 6.6 Hz) at C–28 proton (δC 115.94), a multiplet at 5.35 for C–6 proton (δC 121.70) and a multiplet at 3.53 for C–3 proton (δC 71.80). 13C NMR for both compounds indicated 29 carbons, of which two double bonds and a secondary alcohol (cf exp.) and the DEPT spectra showed that compounds 2 and 3 both contain six methyl, ten methylene, nine methine and four quaternary carbons. The stereochemistry at the side chain double bond can be explained by observing the 1HNMR spectra at the C–25 proton which resonates at δ 2.32 ppm (δC 36.50) in case of (E) isomer and at δ 2.22 ppm (δC 33.90) in case of (Z) isomer (Frost, ward, 1968). The position of the ethylidene group was proved from HMQC, HMBC correlations and COSY experiment. These data are in agreement with literature (John Goad, Akihisa, 1997) which can allow us to assign compound 2 as fucosterol and compound 3 as isofucosterol.
CONCLUSIONS
Phytol (3,7,11,15 tetramethyl–2–hexadec–en–1–ol) is a common diterpenoid in plant kingdom. This report of its isomer 3,7,11,15 tetramethyl–3–hexadec–en–1–ol adds to that on the capability of such red alga of isomerization . In addition, we reported two C29 ethylidene steroids fucosterol and isofucos–terol, which are reported for the first time from the genus Gracilaria.
ACKNOWLEDGMENTS
The authors are thankful to the researcher's assistants for their help in the laboratories work. Deanship of Scientific Research (DSR) is also acknowledged for the financial support (Grant No. 8–003/429).
REFERENCES
Bang, M. H., Chol, S. Y., Jang, T. O., Kim, S. K., Kwon, O. S., Kang, T. C., Won, M. H., Park, J., Baek, N. I. (2002) Phytol, SSADH Inhibitory Diterpenoid of Lactuca sativa. Archives of Pharmacol Research 25: 643– 646. [ Links ]
Chattopadhyay, K., Ghosh, T., Pujol, C. A., Carlucci, M. J., Damonte, E. B., Ray, B. (2008) Polysaccharides from Gracilaria corticata: Sulfation, Chemical characterization and anti–HSV activities. International Journal of Biological Macromolecules 43: 581–588. [ Links ]
Das, B., Srinivas, K. V. N. S. (1992a) Dihydroxysterols from the marine red alga, Gracilaria edulis. Phytochemistry 58: 4371–4373. [ Links ]
Das, B., Srinivas, K. V. N. S. (1992b) Minor C29– steroids from the marine red alga, Gracilaria edulis. Phytochemistry 31: 2427–2429. [ Links ]
Frost, d. J., Ward, J. P. (1968) Stereochemistry of 7, 24(28)–Stigmastadien–3p–ol and the fucosterols. Tetrahedron Letters 34: 3779–3782. [ Links ]
Gibbons, G. F., Goad, L. J., Goodwin, T. W. (1968) The identification of 28– isofucosterol in the marine green algae Enteromorpha intestinalis and Ulva lactuca. Phytochemistry 7: 983–988. [ Links ]
Govindan, M., Hodge, J. D., Brown, K. A., Nunez–Smith, M. (1993) Distribution of cholesterol in caribbean marine algae. Steroids 58: 178–180. [ Links ]
Guiry, M. D., Freamhainn, M. T. (2009) Biosystematics of Gracilari foliifera. Nordic Journal of Botany 5: 629–637. [ Links ]
Hsu, B. Y, Tsao, C. Y., Chiou, T. K., Hwang, P. A., Hwang, D. F. (2007) HPLC determination for prostaglandins from sea weed Gracilaria gigas. Food Control 18: 639–645. [ Links ]
John Goad, L., Akihisa, T. (1977) Analysis of Sterols 1st. eds. Blackie Academic & Proffesional, pp. 382–383. [ Links ]
Kulkarni, S.N., Phadake, A. S., Rangaishenvi, M. V., Kamath, S. V., (1988) Synthesis of phytol isomer 7,11,15–Trimethyl–3–methylenehexadecanol and 3,7,11,15–tetramethyl–3–hexadecen–1–ol and their conversion into vitamin E. Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry 27 B (1): 65–6. [ Links ]
Rodriguez, M. C., Matulewicz, M. C., Nose da, M. D., Ducetti, D. R. B., Leonardi, P. I. (2009) Agar from Gracilaria gracilis (Gracilariales, Rhodophyta) of the patagonic coast of Argentina–Content, structure and physical properties. Bioresource Technology 100: 1435–1441. [ Links ]
Yotsu–Yamashita, M., Abe, K., Seki, T., Fujiwara, K., Yasumoto, T. (2007) Polycarvenoside C and C2, the new analogs of the human lethal toxin polycarvenoside A from the red alga Gracilaria edulis. Tetrahedron Letters 48: 2255–2259. [ Links ]














