Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Boletín de la Sociedad Botánica de México
versión impresa ISSN 0366-2128
Bol. Soc. Bot. Méx no.86 México jun. 2010
Ecología
Post–fire seed bank in a xerophytic shrubland
Banco de semillas después de un incendio en un matorral xerófilo
Yuriana Martínez–Orea1, 3, Silvia Castillo–Argüero1, M. Patricia Guadarrama–Chávez2 and Irene Sánchez1
1 Departamento de Ecología y Recursos Naturales, Facultad de Ciencias, Universidad Nacional Autónoma de México. 3 Autor para la correspondencia: yurimar29@yahoo.com.mx
2 Unidad Multidisciplinaria SISAL Mérida. Facultad de Ciencias Universidad Nacional Autónoma de México.
Received: March 21, 2009.
Accepted: March 15, 2010.
Abstract
Through the seedling emergence method we studied the effects of fire on the soil seed bank of a xerophytic shrubland in two consecutive years. We compared its composition and abundance in two sites, one burned and one unburned. An important proportion of seeds died due to the high temperatures reached by fire. In addition, species richness and, diversity were also negatively affected. These variables showed statistical differences between sites and years. After one year, seed bank abundance and diversity reached higher values. Dominant species were perennial herbs in terms of species number, and in terms of seedling abundance the dominant life form was a tree. However, fire was not a determinant factor in terms of species composition. These results are important to explain the changes in vegetation after a fire, specially if we consider that this site is a natural preserve immersed in an urban area.
Key words: fire, seed bank, xerophytic shrubland.
Resumen
A través de la emergencia de plántulas estudiamos los efectos del fuego en el banco de semillas del suelo de un matorral xerófilo en dos años consecutivos. Comparamos su composición y abundancia en dos sitios, uno quemado y uno no quemado. Una proporción importante de semillas murió debido a las altas temperaturas alcanzadas por el fuego. La riqueza y diversidad de especies fueron negativamente afectadas. Estas variables mostraron diferencias significativas entre sitios y años. Después de un año, la abundancia y diversidad del banco de semillas alcanzaron mayores valores. En términos de riqueza dominaron las hierbas perennes y en términos de abundancia, dominó una especie arbórea. El fuego no fue un factor determinante en la composición de especies. Estos resultados son importantes para explicar los cambios en la vegetación después del fuego, especialmente si consideramos que este sitio es una reserva natural inmersa en un área urbana.
Palabras clave: fuego, banco de semillas, matorral xerófilo.
Seed banks have been documented as highly dynamic and heterogeneous elements in a community (Harper, 1977) representing the space–time changes in composition and abundance of species in a system (Thompson, 1978; Fenner, 1983). These banks influence for example the genetic and species diversity maintenance (Thompson and Grime, 1979). They have also been recognized to be a favorable strategy in habitats under unpredictable environmental conditions (Caballero et al., 2003). Particularly, in shrublands, soil seed banks have been reported as the main source for seedling regeneration (Jiménez and Armesto, 1992), and, in xerophytic shrublands they are known to be abundant for some species (Price and Joyner, 1997; Pazos and Bertiller, 2008), nevertheless, there are several aspects related to seed bank dynamics that still remain little studied.
Composition and abundance of seeds in a soil bank can be affected by intrinsic factors such as dormancy and the specific requirements for germination, as well as by external factors, such as the structure of vegetation, soil characteristics, environmental conditions and the disturbance regime (Baskin and Baskin, 1998).
Particularly, the disturbance regime is a very important factor because, depending on its origin, magnitude, and frequency, it may determine the existence or extinction of seed banks (Middleton, 2003). In xerophytic shrublands, fire is an important disturbance that is part of their natural dynamics (Rodríguez–Trejo, 2008). However, there are also fires caused by human activities that can affect ecological processes related to soil seed banks and vegetation structure. Indeed, in some regions of Mexico there are frequent fires associated to extended drought periods in diverse types of vegetation with a dry season, such as the Pedregal de San Ángel (Juárez–Orozco and Cano–Santana, 2007).Thus, studies that contribute to understand the effect of fire on seed persistence and on seedling emergence are very necessary (Vivar–Evans et al., 2006).
The study site is a natural area immersed in a big city, it represents a unique habitat that harbors 377 plant species (Castillo–Argüero et al., 2009), some are considered as threatened (Flores–Villanueva, 2006), some as endemic (Tellez–Velazco, 2002 in Salazar, 2009; Juárez–Orozco and Cano–Santana, 2007), and others have reduced populations in other parts of the valley of Mexico (Rzedowsky and Calderón, 2001). it offers many environmental services to the city as well (Lot–Helgueras, 2008). But, the dramatic reduction of its original area by urbanization (Cano–Santana et al., 2008), the occurrence of fires (Juárez–Orozco and Cano–Santana, 2007), the collection of several species for ornamental use (Salazar, 2009), garbage deposition (Cano, pers. obs. 2009) and the presence of invasive and or weed species diaspores in the seed rain constantly threat this system (Castillo–Argüero et al., 2009). Therefore studies that reveal the reliance of species on the soil seed bank for the maintenance of their populations are important. Thus, this research evaluated experimentally the changes in composition and abundance of the soil seed bank in a xerophytic shrubland in the Pedregal de San Ángel, Mexico City, immediately after an anthropic fire and one year after, comparing burned sites with unburned sites as control. We expect smaller values of abundance and species richness in the burned site, and larger values of these variables in the unburned site; and specially in both sites one year after the fire due to a recovery of the soil seed bank.
Materials and Methods
Study site. The ecological reserve "El Pedregal de San Ángel" is a xerophytic shrubland that comprises an area of 237.3 ha (De la Fuente, 2005). it is located at the southwest of Mexico City (19°18'31"–19°19'17" N, 99°10'20"–99°11'52" W), at an altitude of 2200–2277 m asl. The substrate consists of a thick lava layer that was originated by the eruption of the Xitle volcano 2000 years ago (Martin del Pozzo, 1995). The soil layer is usually very thin, it's origin is organic and eolic and retains little humidity. A peculiar feature of the Pedregal de San Ángel is that it shows a high environmental heterogeneity provided by a contrasting topography, and by very marked seasonal changes (Cano–Santana, 1994). The climate is temperate humid, with a mean annual temperature ranging from 14 to 17°C, and a mean annual precipitation of 870 mm (García, 1988), the driest months are December, January and February (Carrillo–Trueba, 1995). The most diverse plant families, are: Asteraceae, Poaceae, and Fabaceae (Castillo–Argüero et al., 2004). The vegetation was frequently affected by fires that occurred mainly during the dry season (February–April), mainly of anthropic origin. Recently, between 1992 and 1997, there were 455 fires in this area and nearby sites (Juárez–Orozco and Cano–Santana, 2007).
Sampling design. The seed bank species composition was analyzed through the seedling emergence method. Two sites were located, one in a conserved area, and one more in an area that was affected by a surface fire that occurred in March 1998. Six days after this disturbance, a 100 × 100 m plot was established in each site. Within each plot, we randomly set a sample of 100 points where we collected 300 g of soil in quadrats of 20 × 20 cm. Collected soil was dried at room temperature for three days. Each soil unit was placed in a pot (20 cm × 20 cm and 8 cm depth). All pots were placed in a greenhouse located nearby the study site following a design of 100 soil pots × 2 sites. A vinylon cloth (mesh size = 2mm) was used to isolate the soil pots from other seeds in the greenhouse. Once seedlings started to emerge, they were harvested every two weeks along one year. All seedlings were transplanted to bigger pots for further determination when possible. This experimental procedure was repeated one year after in the same month (March 1999). Data analyses. We calculated the Chao–Sørensen similarity index through the Estimates program (Colwell, 2005), in order to compare seed bank composition between sites and years. We also calculated Shannon's Diversity and Equitability indexes with the same program. We carried out a bifactorial ANOVA to test the effects of site (burned, unburned) and time (between years) on the seed bank richness, abundance, diversity and equitability. We finally compared differences among soil samples by a Tukey test. All data were logarithmic transformed (Zar, 1990). Seedling density per soil unit area (cm2) was calculated for each site and year.
Results
Seed bank size and species features. We obtained a total of 1130 seedlings in the burned site in 1998, which correspond to thirty seven species; from these, twenty seven were herbs, nine shrubs and one tree species. in the 1998 unburned site, we registered 4325 seedlings, distributed in forty three species, thirty five herbs, seven shrubs and one tree species. in 1999, we found in the burned site 2719 seedlings belonging to forty four species, thirty two herbs, ten shrubs and two tree species, whereas in the unburned site, we had a total of 3686 seedlings, corresponding to forty five species, thirty two herbs, eleven shrubs and two tree species.
Based on seedling density values, the highest corresponded to the unburned site in 1998 with 14 seedlings/m2. in contrast, the smallest value of seedling density for the same year was 3.6 seedlings/m2 for the burned site. One year after the fire, in 1999, seedling density slightly decreased in the unburned site (12 seedlings/m2), and increased in the burned site (9 seedlings/m2). In terms of the number of species, the most represented family was the Asteraceae, for both sites and years; however, based on the number of seedlings, the family with the highest number of seedlings was the Loganiaceae (Buddleia cordata).
According to the life histories of species, we found a 75% of perennials and a 25% of annuals in the unburned site, for the two consecutive years. In the burned site we found similar values: 80% of perennials and 20% of annuals, for both years.
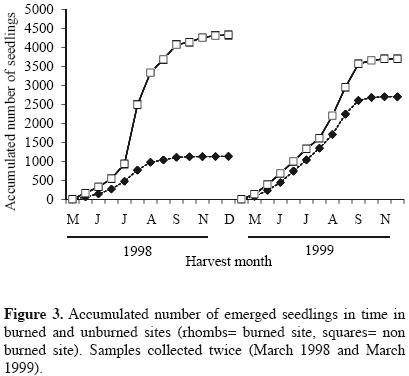
Approximately 180 days after the experiment started, the highest seedling emergence occurred in both sites for the harvests corresponding to the months from August to october, in 1998 and 1999. In these months, most of the seedlings corresponded to Buddleia cordata, Muhlenbergia robusta, Gamochaeta americana and Montanoa tomentosa, the latter was particularly more abundant in the burned site with respect to the unburned site. The highest seedling abundance always occurred in the unburned site for both years. In 1999, curves of seedlings abundances of burned and unburned sites appeared closer (Figure 1, Table 1). Species number per harvest date was also higher for the unburned site in the first year. One year after the fire, the number of species increased in the two sites (Figure 2). Seedling emergence slowly increased in a constant rate after September, and the curves got to a steady state by November for both cases (Figure 3). The number of accumulated species reached a plateau by September in both sites in 1998. For the second year this occurred by August. Both sites appeared closer in their richness values one year after the fire, with a recovery in the burned site (Figure 4).




Abundance, richness, diversity and equitability. Species richness and seedling abundance in the burned site in the year of the fire were significantly smaller than in the unburned site (Table 1). The ANOVA test showed significant differences in seedling abundance due to the fire (F = 109.87, df = 396, p<0.001) and time (F = 54.66, df = 396, p<0.001) factors, levels with the highest mean values were the unburned site and the 1999 year, respectively. Also, the interaction of both factors was significant (F = 27.80, df = 396, p<0.001), the groups with the highest mean values were as well, the unburned site and the year after the fire (1999). Seedling richness showed statistical differences between sites (F = 77.09, df = 396, p<0.001) and years (F = 53.16, df = 396, p<0.001) as well; the interaction of these factors affected significantly the seed bank species richness (F = 77.09, df = 396, p<0.001). Between years, there were also global significant differences among these variables, having higher values in the year after the fire (Table 1).
Diversity and equitability indexes (Table 2) showed the same trend for the fire factor (sites) and the year factor (1998 and 1999). The unburned site showed significantly higher values of these variables (H'= 2.49, E = 0.7) than the burned site (H'= 2, E = 0.5) in the year of the fire, whereas species diversity and equitability increased their values one year after the fire in the burned site (H' = 2.5, E = 0.7). The ANOVA test showed significant differences in the seed bank diversity due to the fire (F = 24.95, df = 396, p<0.001) and time (F = 15.224, df = 396, p <0.001) factors, as well as by the interaction of both (F = 7.5, df = 396, p<0.001). Seed bank equitability showed differences between sites (F = 16.13, df = 396, p<0.001) and years (F = 11.09, df = 396, p<0.001), differences were also evident due to the interaction between fire and time factors (F = 6.68, df = 296, p<0.001). in both cases, levels with the highest mean values were the unburned site, and the year after the fire (1999). Similarity Index. Most of the similarity index values were relatively high (above 50%) in both sites and years, which reflects a similar composition in the seed banks. The highest similarity index value (0.70) occurred between sites in the year of the fire, whereas, the lowest similarity index value (0.58) was obtained when comparing the 1998 burned site and the 1999 unburned site.

Discussion
Seedling abundance between burned and unburned studied sites in the Pedregal de San Ángel showed clear differences. There was a lower seedling emergence in the burned site than in the unburned one immediately after the fire (Table 1); similar results have been obtained in other studies carried out in shrublands, where there was a substantial decrease of seeds in seed banks after a fire (Pierce and Cowling, 1991; Ferrandis et al., 1999). Basically the significant smaller seed bank values in the burned site can be explained by the high temperatures reached by fire, as it has been reported by Turna and Bilgili (2006), who mentioned that exposure to temperatures between 110°C and 150°C had negative effects on germination percentages in some species in temperate sites. Tesfaye et al. (2004) reported as well a strong reduction in the size of seed banks, where many seeds that were not deeply buried in the soil died. Keeley and Keeley (1987) have as well suggested that a heat treatment reduces germination in some chaparral species and Legg et al. (1992) explained this result in terms of a complete seed combustion and/or embryo death, which cause a serious depletion of the seed bank specially in the upper 2–3 cm of soil in some shrubland species. in addition, ashes have been reported to produce high pH soil values that may inhibit seed germination of some species (Ne'eman et al., 2009). In a temperate forest Luzuriaga et al. (2005) found out that some anthropic disturbances, which include fire, can lead to an important decrease of about 75% of seed abundance in seed banks. Esposito et al. (2006) and Crosti et al. (2006) found as well a strong decrease in seedling emergence caused by high intensity fires in mediterranean environments. Therefore we suggest that fire is a disturbance that can cause an important negative effect in terms of seed mortality in the seed bank of a xerophytic shrubland, although for some species fire might have a positive effect (Valbuena and Trabaud, 2001; Pérez–Fernández and Rodríguez–Echeverría, 2003; Ne'eman et al., 2009).
The presence of a thick testa in the seed has been proved to protect the embryo from high temperatures which can be lethal for seeds stored in the soil (Herranz et al., 1998). Soil seed bank species richness, diversity and equitability were significantly smaller in the burned site in the year of the fire, where seed mortality probably experienced the largest values due to the lack of a thick testa (Baskin and Baskin, 1998; Castillo–Argüero et al., 2001). This was evident in some species of the Asteraceae, orchidaceae and Poaceae families, which showed smaller abundances in the burned site. In addition, fire could have affected positively other species that require elevated temperatures for dormancy breaking (Herranz et al., 1998), such as Dodonaea viscosa (Baskin and Baskin, 1998) since this species showed higher seedling abundances in the burned site. The higher abundances in the burned site –right after the fire– of some species that lack a thick testa, such as Montanoa tomentosa, could be explained differently. Some substances released from burned vegetation, such as ethylene and some nitrogen compounds are implicated in fire–triggered germination for some Asteraceae (Macchia et al., 2001). Furthermore other studies suggest that oxidizing gases and acids generated in smoked and charred wood are responsible for triggering germination (Keeley and Fotheringham, 1998) in some chaparral and woodland species (Pérez–Fernández and Rodríguez–Echeverría, 2003).
Seedling abundance increased in the burned site in 1999. After a fire, there is an important release of nutrients, which may have improved species establishment (Rai and Tripathi, 1987; Pausas et al., 2003); also, an induction of flowering might be caused by fire in some species (Brewer, 1995). In this sense we may expect a post fire seed bank recovery in this site, specially because values of diversity, equitability, and species richness increased one year after the fire. Ferrandis et al. (1999) reported a dramatic seed bank depletion by fire (more than 90% for some species). Nevertheless there was a significant increase in seedling abundance confined to the first post–fire year in a Mediterranean shrubland, like in this case.
In general, temporal and spatial changes in seed bank species composition and abundance are probably due to the natural year to year variation in seed production and dispersal in all communities. Particularly in the Pedregal de San Ángel, Cano–Santana (1994) found different patterns of vegetative and reproductive allocation among species. These differences may directly influence the composition and abundance of the seed rain, which represents the potential contribution to the seed bank. More recently, Camacho (2007) studied the dynamics of the seed rain in this community and reported a high heterogeneity in the abundance and richness among seasons and between burned and unburned sites. Specifically, in sites that had been burned, this author reported higher seed rain densities compared to his control sites, and also a great abundance of some particular species, such as Buddleia cordata.
Species similarity was relatively high in all comparisons, despite the statistical differences in richness and abundance. Seed bank composition was similar in both sites and years, probably as a result of the annual seed production and of the dispersal ability of all species in the area, most of them anemochorous (César–García, 2002). This points out the existence of a constant supply of diaspores to the seed bank, which explains the presence of most of the species in both sites and years. It also suggests that this seed bank probably includes three types of species: those that do not survive fire, those that are fire resistant and those that are stimulated to germinate by fire, as it has been seen in some Mediterranean ecosystems (Paula and Pausas, 2008). In addition, similarity value between burned and unburned sites increased one year after the fire, suggesting again that some species recovered and probably increased their seed production. This recovery may be explained in part by the resprout–ing of the vegetation and by the increase in flowering and fruiting after a fire showed by some shrubland species (Kalamees and Zobel, 2002) which could have contributed to a greater abundance of the soil seed bank one year after the fire. It has been recognized that vegetation recovery relies greatly on buried seeds (Kalamees and Zobel, 2002), nevertheless it is important to find out the long term effects of fire in this community, where most of species are perennials. With regards to the soil seed bank species in the burned site one year after the fire and their biological traits, most were herbs, with a dominance of perennials over annuals, with an important proportion of species that disperse through ane–mochory. Valbuena and Trabaud (2001) reported the same pattern when studying the contribution of the soil seed bank to post–fire recovery in a Mediterranean shurbland.
With respect to annual species it is important to consider that some of them are more affected by fire since their permanence relies in their annual seed production, and still if some surviving species are not therophytes, their below ground perennating organs can show as well severe damage by fire (Mallik et al., 1984). These processes lead to the need for more studies that reveal the long term responses of these species to disturbances such as fire, which becomes a priority specially if we consider that endemic or exclusive to shrubland vegetation species were present in low abundances or were absent in the seed bank of the burned site.
In shrublands, there is a mixture of sprouters and obligate seeders (Valbuena and Trabaud, 2001). Therefore, after a fire, regeneration potential of vegetation in the Pedregal de San Ángel will rely on a mixture of this kind of species. Those species that arrive as propagules through dispersal, as well as growth and seed production of those which survive in the site, are in charge of the maintaining of species within their community. Soil seed bank recovery will be affected by these processes as well. Species which are stimulated by fire to germinate, to resprout, or to produce higher amounts of seeds will contribute greater to the seed bank of this site along time. Those species that do not survive fire will be less abundant in the following regeneration stages.
The rate of soil seed bank turnover depends on the balance between several factors such as seed production, dispersal, dormancy and germination (Pake and Venable, 1996) as well as on the disturbance regime (Milberg, 1995). These factors vary in spatial and temporal scales (Harper, 1977). Temporally, seeds are added to the soil in periods between fire events (Delgado et al., 2008; Keeley and Keeley, 1987), this is why some studies address the seed bank dynamics in relation to stand age since the last fire (Clemente et al., 2007). The present research offers useful information in terms of the seed bank composition and abundance ten years ago, when fires used to be frequent in this reserve (Martínez–Mateos, 2001; Martínez–Orea, 2001; Juárez–Orozco and Cano–Santana, 2007). The availability of previous data is in consequence essential for an evaluation of the entrance, permanence or absence of species in the soil seed bank of this natural reserve in time.
Acknowledgements
We thank two anonymous reviewers of previous versions of this paper for their suggestions and comments. Specially, we thank Silvia Iriarte–Vivar for the manuscript revision and language edition. We are grateful to Marco Antonio Romero–Romero, Eduardo Pérez–García, Oswaldo Núñez and Edith Martínez for their valuable field and green house assistance. This study was partly supported by a grant from Fundación UNAM and PAPIME Project No. 182033.
Literature Cited
Baskin Ce. and Baskin J.M. 1998. Seeds, Ecology, Biogeography, and Evolution of Dormancy and Germination. Academic Press. U.S.A. [ Links ]
Brewer S.J. 1995. The relationship between soil fertility and fire–stimulated floral induction in two populations of grass–leaved golden aster, Pityopsis graminifolia. Oikos 74:45–54. [ Links ]
Caballero I.J., Olano J.M., Loidi J. and Escudero A. 2003. Seed bank structure along a semi–arid gypsum gradient in Central Spain. Journal of Arid Environments 55:287–299. [ Links ]
Camacho A.J.M. 2007. Efecto del fuego sobre la lluvia de semillas en la Reserva del Pedregal de San Ángel, México D.F. Bachelor Thesis, Facultad de Ciencias, Universidad Nacional Autónoma de México. Mexico. 53 pp. [ Links ]
Cano–Santana Z. 1994. Flujo de energía a través de Sphenarium purpurascens (Orthoptera: Acrididae) y productividad primaria neta aérea en una comunidad xerófita. Ph.D. Dissertation , Instituto de Ecología, Universidad Nacional Autónoma de México, Mexico, D.F. 198 pp. [ Links ]
Cano–Santana Z., Castillo–Argüero S., Martínez–Orea Y., Juárez–Orozco S. 2008. Análisis de la riqueza vegetal y el valor de conservación de tres áreas incorporadas a la Reserva Ecológica del Pedregal de San Ángel, Distrito Federal (México). Boletín de la Sociedad Botánica de México 82:1–14. [ Links ]
Carrillo–Trueba C. 1995. El Pedregal de San Ángel. Universidad Nacional Autónoma de México. Mexico, D.F. [ Links ]
Castillo–Argüero S., Guadarrama–Chávez M.P., Martínez Y., Mendoza–Hernández PE., Núñez–Castillo O., Romero–Romero M.A., and Sánchez–Gallén I. 2002. Diásporas del Pedregal de San Ángel. Facultad de Ciencias. UNAM. Mexico, D.F. [ Links ]
Castillo–Argüero S., Montes–Cartas G., Romero–Romero M.A., Martínez–Orea Y., Guadarrama–Chavez M.P, Sánchez–Gallén I. and Núñez–Castillo O. 2004. Dinámica y Conservación de la Flora del Matorral Xerófilo de la Reserva Ecológica del Pedregal de San Ángel (D.F., México). Boletín de la Sociedad Botánica de México 74:51–75. [ Links ]
Castillo–Argüero S., Martínez–Orea Y., Meave J.A., Hernández–Apolinar M., Núñez–Castillo O., Santibáñez–Andrade G. and Guadarrama–Chavez M.P. 2009. Flora: susceptibilidad de la comunidad a la invasión de malezas nativas y exóticas. In: Lot A. and Cano–Santana Z. eds. Biodiversidad del ecosistema del Pedregal de San Ángel. p.107–117. Universidad Nacional Autónoma de México, Mexico, D.F. [ Links ]
César–García S.F. 2002. Análisis de algunos factores que afectan la fenología reproductiva de la comunidad vegetal de la Reserva del Pedregal de San Ángel, D.F. (México). Bachelor Thesis, Facultad de Ciencias, Universidad Nacional Autónoma de México, Mexico, D.F. 112 pp. [ Links ]
Clemente A.S., Rego F.C. and Correia O.A. 2007. Seed bank dynamics of two obligate seeders, Cistus monspeliensis and Rosmarinus officinalis, in relation to time since fire. Plant Ecology 190:175–188. [ Links ]
Colwell R.K. 2005. Estimates v7.5.: statistical estimation of species richness and shared species from samples. <viceroy.eeb.uconn.edu/EstimateS> [ Links ]
Crosti R., Ladd P.G., Dixon K.W. and Piotto B. 2006. Post–fire germination: The effect of smoke on seeds of selected species from the central Mediterranean basin. Forest Ecology and Management 221:306–312. [ Links ]
Dansereau P, and Lems, K. 1957. The grading of dispersal types in plant communities and their ecological significance. Conti–butions de L'Institute Botanique de L'Université de Montreal 71:5–52. [ Links ]
De la Fuente R. 2005. Acuerdo por el que se rezonifíca, delimita e incrementa la zona de la Reserva Ecológica del Pedregal de San Ángel de Ciudad Universitaria. Gaceta UNAM, Universidad Nacional Autónoma de México 3813:14–15, 20–21 p. [ Links ]
Delgado J.A., Serrano J. M., López F. and Acosta F.J. 2008. Seed size and seed germination in the Mediterranean fire–prone shrub Cistus ladanifer. Plant Ecology 197:269–276. [ Links ]
Esposito A., Strumia S., Caporaso S. and Mazzoleni S. 2006. The effect of fire intensity on soil seed bank in Mediterranean macchia. Forest Ecology and Management 234S:S207. [ Links ]
Fenner M. 1983. Relationships between seed weight, ash content and seedling growth in twenty–four species of Compositae. New Phytologist 95:697–706. [ Links ]
Ferrandis P., Herranz J.M. and Martínez–Sánchez J.J. 1999. Effect of fire on hard–coated Cistaceae seed banks and its influence on techniques for quantifying seed banks. Plant Ecology 144:103114. [ Links ]
Flores–Villanueva L. 2006. Contribución al estudio de la Familia Orchidaceae en la Reserva del Pedregal de San Ángel y en algunas zonas perturbadas por la urbanización de la Ciudad Universitaria UNAM, México, D.F. Bachelor Thesis, Facultad de Ciencias, Universidad Nacional Autónoma de México, Mexico, D.F., 120 pp. [ Links ]
García E. 1988. Modificaciones al Sistema de Clasificación Climático de Köppen para adaptarlo a las condiciones de la Republica Mexicana, México. Instituto de Geografía, UNAM, Mexico, D.F. [ Links ]
Harper J.L. 1977. The population Biology of plants. Academic Press. London. [ Links ]
Herranz J.M., Ferrandiz P. and Martínez–Sánchez J.J. 1998. Influence of heat on seed germination of seven Mediterranean Leguminosae species. Plant Ecology 136:95–103. [ Links ]
Jiménez H. E. and Armesto J.J. 1992. Importance of the soil seed bank of disturbed sites in Chilean matorral in early secondary succession. Journal of Vegetation Science 3:579–586. [ Links ]
Juárez–Orozco S. and Cano–Santana Z. 2007. El cuarto elemento y los seres vivos: ecología del fuego. Ciencias 85:4–12. [ Links ]
Kalamees R. and Zobel M. 2002. The role of the seed bank in gap regeneration in a calcareous grassland community. Ecology 83:1017–1025. [ Links ]
Keeley J.E. and Keeley S.C. 1987. Role of fire in the germination of chaparral herbs and suffrutescents. Madroño 34:240–249. [ Links ]
Keeley J.E. and Fotheringham C.J. 1998. Smoke–induced seed germination in California chaparral. Ecology 79:2320–2336. [ Links ]
Legg C.J., Maltby E. and Proctor M.C.F. 1992. The ecology of severe moorland fire on the North York Moors: Seed distribution and seedling establishment of Calluna vulgaris. Journal of Ecology 80:737–752. [ Links ]
Lot–Helgueras A. 2008. 25 años de la reserva del Pedregal de San Ángel. Ciencias 91:30–32. [ Links ]
Luzuriaga A.L., Escudero A., Olano J.M. and Loidi J. 2005. Regenerative role of seed banks following an intense soil disturbance. Acta Oecologica 27:57–66. [ Links ]
Macchia M., Angelini L. and Ceccarini L. 2001. Methods to overcome seed dormancy in Echinacea angustifolia DC. Scientia Horticulturae 89:317–324. [ Links ]
Mallik A.U., Hobbs R.J. and Legg C.J. 1984. Seed dynamics in Calluna–Arctostaphylos heath in north–eastern Scotland. Journal of Ecology 72:855–871. [ Links ]
Martin–del–Pozzo. 1995. La edad del Xitle. In: Carrillo–Trueba C. 1995. El Pedregal de San Angel p. 48 Universidad Nacional Autónoma de México, México, D.F. [ Links ]
Martínez–Mateos A.E. 2001. Regeneración natural después de un disturbio por fuego en dos microambientes contrastantes de la Reserva Ecológica "El Pedregal de San Ángel". Bachelor Thesis, Facultad de Ciencias, Universidad Nacional Autónoma de México, Mexico, D.F., 66 pp. [ Links ]
Martínez–Orea Y. 2001. Efecto del fuego sobre el banco de semillas de la Reserva Ecológica del Pedregal de San Ángel. Bachelor Thesis, Facultad de Ciencias, Universidad Nacional Autónoma de México, Mexico D.F., 62 pp. [ Links ]
Middleton B.A. 2003. Soil seed banks and the potential restoration of forested wetlands after farming. Journal of Applied Ecology 40:1025–1034. [ Links ]
Milberg P. 1995. Soil seed bank after eighteen years of succession from grassland to forest. Oikos 72:3–13. [ Links ]
Ne'eman G., Ne'eman R., Keith D.A. and Whelan R.J. 2009. Does post–fire plant regeneration mode affect the germination response to fire–related cues? Oecologia 159:483–492. [ Links ]
Pake C.E. and Venable D.L. 1996. Seed banks in desert annuals: Implications for persistence and coexistence in variable environments. Ecology 77:1427–1435. [ Links ]
Paula S. and Pausas J.G. 2008. Burning seeds: germinative response to heat treatments in relation to resprouting ability. Journal of Ecology 96:543–552. [ Links ]
Pausas J.G., Ouadah N., Ferran A., Gimeno T. and Vallejo R. 2003. Fire severity and seedling establishment in Pinus halepensis woodlands, eastern Iberian Peninsula. Plant Ecology 169:205213. [ Links ]
Pazos G.E. and Bertiller M.B. 2008. Spatial patterns of the germinable soil seed bank of coexisting perennial–grass species in grazed shrublands of the Patagonian Monte. Plant Ecology 198:111–120. [ Links ]
Pérez–Fernández M.A. and Rodríguez–Echeverría S. 2003. Effect of smoke, charred wood and nitrogenous compounds on seed germination of ten species from woodland in central–western Spain. Journal of Chemical Ecology 29:237–251. [ Links ]
Pierce M.S. and Cowling M.R. 1991. Dynamics of soil–stored seed banks of six shrubs in fire–prone dune fynbos. Journal of Ecology 79:731–747. [ Links ]
Price M.V. and Joyner J.W. 1997. What resources are available to desert granivores: seed rain or soil seed bank? Ecology 78:764773. [ Links ]
Rai J.P.N. and Tripathi R.S. 1987. Germination and plant survival and growth of Galinsoga parviflora Cav. as related to food and energy content of its ray– and discachenes. Acta Oecologica/ Oecologia Plantarum 8:155–165. [ Links ]
Raunkiær, C. 1978. The Life Forms of Plants. Oxford University Press, Oxford. [ Links ]
Rodríguez–Trejo D.A. 2008. Fire regimes, fire ecology, and fire management in Mexico. Ambio 37:548–556. [ Links ]
Rzedowsky J. 2001. Extinción de especies vegetales. In Rzedowsky J. and Rzedowsky G.C. eds. Flora Fanerogámica del Valle de México. 30–32 pp. CONABIO, Instituto de Ecología, A.C., Pátzcuaro, Michoacán, Mexico. [ Links ]
Salazar G.A. 2009. Orquídeas. In: Lot A. y Cano–Santana Z. eds Biodiversidad del ecosistema del Pedregal de San Angel p.153–169. Universidad Nacional Autónoma de México, Mexico, D.F. [ Links ]
Tesfaye G., Teketay D., Assefa Y. and Fetene M. 2004. The Impact of Fire on the Soil Seed Bank and Regeneration of Harenna Forest, Southeastern Ethiopia. Mountain Research and Development 24:354–361. [ Links ]
Thompson K. 1978. The occurrence of buried viable seeds in relation to environmental gradients. Journal ofBiogeography 5:425–430. [ Links ]
Thompson K. and Grime J. P. 1979. Seasonal variation in the seed banks of herbaceous species in ten contrasting habitats. Journal of Ecology 67:893–921. [ Links ]
Turna I. and Bilgili E. 2006. Effect of heat on seed germination of Pinus sylvestris and Pinus nigra ssp. pallasiana. International Journal of Wildland Fire 15:283–286. [ Links ]
Valbuena L. and Trabaud L. 2001. Contribution of the soil seed bank to post–fire recovery of a heathland. Plant Ecology 152:175–183. [ Links ]
Vivar–Evans S., Barradas V.L., Sáchez–Coronado M.E., Gamboa de Buen A. and Orozco–Segovia A. 2006. Ecophysiology of seed germination of wild Dahlia coccinea (Asteraceae) in a spatially heterogeneous fire–prone habitat. Acta Oecologica 29: 187–195. [ Links ]
Zar J. 1990. Biostastical Analysis. Prentice Hall. Englewood Cliffs. New Jersey. [ Links ]














