Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Boletín de la Sociedad Botánica de México
versión impresa ISSN 0366-2128
Bol. Soc. Bot. Méx no.85 México dic. 2009
Taxonomía y florística
The Dryopteris patula complex (Dryopteridaceae) in Mexico: morphometric analyses
El complejo Dryopteris patula (Dryopteridaceae) en México: análisis morfométricos
Victoria Hernández–Hernández1, 3, Teresa Terrazas2 and Claudio Delgadillo Moya2
1 Programa en Botánica, Colegio de Postgraduados. Montecillo, Estado de México, 56230, México.
2 Departamento de Botánica. Instituto de Biología, Universidad Nacional Autónoma de México, Apartado Postal 70–233, México, D.F. 04510, México.
3 Corresponding author. Present address: Departamento de Ecología Funcional, Instituto de Ecología, A.C. Km 2.5 antigua carretera a Coatepec No. 351, Congregación El Haya, Xalapa 91070, Veracruz, México. E–mail: victoria.hernandez@inecol.edu.mx
Received: may 25, 2009.
Accepted: october 13, 2009.
Abstract
We studied collections from four species of the Dryopterispatula complex (D. cinnamomea, D. patula, D. rosea, and D. rossii) to identify the morphological characters that distinguish them from one another. D. maxonii and D. wallichiana were included as comparative species to evaluate characters that distinguish species within the complex. Quantitative characters were examined through principal component and canonical discriminant analyses, and both qualitative and quantitative characters were used to obtain phenograms. Multivariate analyses determined that basal pinna length, stipe scale length, number of pinna pairs, and frond length are the variables that discriminate among D. rossii and the other species of the complex. The phenogram showed two groups. One included D. maxonii and D. wallichiana, while the second grouped the four species of the complex. These species were distinguished by the shape and margin of their rhizome scales, color of stipe, and shape of the blade. A combination of morphological characters supports the recognition of the four species as valid. An identification key is given and a taxonomic treatment is presented for each of the taxa.
Key words: discriminant analysis, Dryopteris cinnamomea, Dryopteris rosea, Dryopteris rossii, phenogram, pinnae, scales.
Resumen
Se estudiaron las especies del complejo Dryopteris patula (D. cinnamomea, D. patula, D. rosea y D. rossii) con el objetivo determinar los caracteres morfológicos con valor taxonómico que permitan distinguirlas. También se incluyó a D. maxonii y D. wallichiana como especies que representan un grupo de referencia para confirmar los caracteres de los taxa del complejo. Los caracteres cuantitativos se analizaron a través de los análisis componentes principales y discriminante canónico y los cualitativos y cuantitativos para generar los fenogramas. El número de pinnas, largo de la pinna basal y largo de las escamas del pecíolo son variables que permiten distinguir a D. rossii de las otras especies del complejo. El fenograma mostró dos grupos: el primero corresponde a las especies de referencia, mientras que el segundo a las especies del complejo las cuales se distinguen por la forma y margen de las escamas del rizoma, color de pecíolo y forma de la fronda. Las cuatro especies se reconocen por una combinación única de caracteres. Se presenta una clave para su identificación y el tratamiento taxonómico para cada taxon.
Palabras clave: análisis discriminante, Dryopteris cinnamomea, Dryopteris rosea, Dryopteris rossii, fenograma, escamas, pinnas.
The genus Dryopteris is represented by thirteen species in Mexico (Mickel and Smith, 2004). Dryopteris has various taxonomic complexes, on the grounds that the genus presents characters that vary so broadly that it is not possible to distinguish between species. For example, Mickel and Beitel (1988) defined two complexes in need of revision. The first, which is here referred to as the Dryopteris wallichiana complex includes D. wallichiana (Spreng.) Hyl., and D. pseudofilixmas (Feé) Rothm. The second, which we refer to as the Dryopteris patula complex includes, five taxa: D. cinnamomea (Cav.) C. Chr., D. patula (Sw.) Underw., D. rosea (E. Four.) Mickel & Beitel, D. rossii C. Chr., and D. simplicior Mickel & Beitel. These species share characters such as the presence of glands on the blade and the size of the rhizome scales. However, numeric discontinuities have not been found in these morphological attributes. Moran (1995) argued for the need to study D. patula, which likely involves at least three species, as judged by the wide variability in the density of glands on the blade, the size of scales on the stipe, and the leaf dissection. Moran does not, however, recognize the complex proposed by Mickel and Beitel (1988). Riba and Pérez–García (1999) also considered D. patula to be associated with the group consisting of D. cinnamomea, D. rosea and "others," which they do not specify. These associations are based on the size of scales of the rhizome, blade indument, and color overlapping of axes. Both Moran (1995) and Riba and Pérez–García (1999) placed D. simplicior as a synonym of D. patula; thus according to their concept the D. patula complex is composed of four species: D. cinnamomea, D. patula, D. rosea, and D. rossii. More recently, Mickel and Smith (2004) claim that D. rosea is a synonym of D. cinnamomea and describe D. knoblochii A. R. Sm. as a new species in the complex from material previously determined as D. cinnamomea, D. rossii and D. patula var. rossii. These authors also suggest the need of further work to understand the species of this complex (D. cinnamomea, D. knoblochii, D. patula, and D. rossii) of low to middle elevations, and why different authors recognized different number of species.
Multivariate analyses of ferns and lycophytes have been used to explore and determine characters relevant to separate species groups and to support the recognition of species (Paris and Windham, 1988; Sato and Tsuyuzaki, 1988; Sato, 1989; Mayer and Mesler, 1993; Speer and Hilu, 1999; Small and Hickey, 2001). Some characters included in multivariate analyses for segregating ferns and lycophytes species are blade, stipe, and petiolule features, the shape of rachis scales, the indusial margin, the number of sporangium cells, and the color, shape, and diameter of spores (Waterway, 1986; Paris and Windham, 1988; Mayer and Mesler, 1993; Speer and Hilu, 1999; Small and Hickey, 2001; Lee et al., 2006). The main objective of this study was to use multivariate analyses to recognize the morphological characters with taxonomic value for delimiting species in the Dryopteris patula complex.
Materials and Methods
More than 430 specimens of the Dryopteris patula complex were studied, including D. cinnamomea, D. patula, D. rosea, and D. rossii. Samples were collected in the field in the states of Guerrero, México, Michoacán, Morelos, and Veracruz or obtained via herbarium specimens (from CHAPA, ENCB, IEB, and MEXU, a complete list may be requested from the correspondence author). Only 144 specimens were suitable for the analyses (see in descriptions and Appendix) because all organs were present. D. knoblochii was not included in the study because only two specimens were deposited in the herbaria and their fronds were incomplete. We were unable to collect this species in the field. Only one specimen of D. simplicior was deposited in the herbaria cited above . This sample was collected in Villa de Flores, Chiapas by M. A. Pérez 603, and only part of the pinna was preserved and not included in the analyses.
Twenty four qualitative and 16 quantitative characters were evaluated (Table 1). These included characters of rhizome, stipe, rachis, blade, pinna, 3rd order segments, sori, and indusium. The terminology follows Pérez–García and Riba (1990). Type specimens of the taxa studied were obtained from herbaria (NY, P, S), and observations were recorded from their photographs (see in descriptions). However, some characters like indument on blade, axes, and scales were omitted because it was not possible to observe them in the photographs.

Dryopteris maxonii and D. wallichiana were included as comparative species for this study to corroborate the variability of characters within the species D. patula complex. Both species have distinctive characters unshared with the species of the D. patula complex such as frond dissection, number of pinna pairs, indusia shape, and lack glands. D. wallichiana is grouped into the D. wallichiana complex, whereas D. maxonii has never been associated with the species of the complex studied.
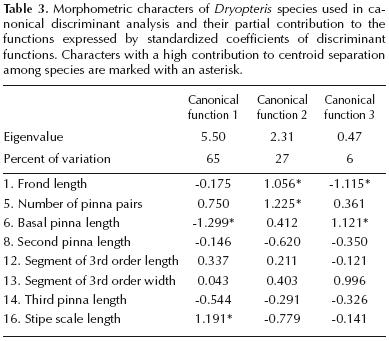
Statistical analyses. Quantitative variables were natural logarithm transformed, except for number of pinna pairs square–root transformed to carry out the analyses. First, in order to reduce the number of variables, a principal component analysis (PCA) was conducted, using the sixteen quantitative variables (Table 2), as an exploratory analysis without establishing any particular hypothesis (Johnson, 2000). The eight characters with the highest eigenvectors in PCA were used to perform a canonical discriminant analysis (CANDISC) under the hypothesis that differences among groups (species) will be maximized and that discriminating characters will allow recognition of the groups (Johnson, 2000) and the Mahalanobis distances were calculated to evaluate if statistical differences exist between pairs of species. in addition, the characters with the greatest weight in CANDISC were tested in a variance analysis (GLM) in order to confirm statistically significant differences among species and to establish character states. All analyses were conducted with SAS statistical package version 6.03 (SAS Institute 1989).

Two similarity analyses were conducted using a matrix of 144 individuals plus the four type specimens. All character states were coded as binary independent characters (Crisci and López–Armengol, 1983). In the first analysis 98 characters (Table 1) were analyzed. The second analysis was conducted with 84 characters, including only three quantitative characters (number of pinna pairs, basal pinna length and stipe scales length) highlighted by CANDISC. Similarity matrixes were obtained using the Jaccard index. This index is defined as the intersections over the total number of characters minus the number of non–intersections. Phenograms were produced by the average linkage of the unweighted arithmetic mean, UPGMA, which selects the average similarity values from the individuals and does not eliminate any value (Crisci and López–Armengol, 1983).
Results
Statistical analyses. PCA showed that the first three components explain 86 % of the total variation, the first component explaining 65 %. The variables with most information were length of the secondary pinna (8), frond length (1), basal pinna length (6), and the third pinna length (14). The second component explained a further 15 %, and the variables with higher eigenvectors were stipe scale length (16), segment of 3rd order width (13), and number of pinna pairs (5). The third component explained 6 % of the remaining variance, the most important character being segment of 3rd order length (12) (Table 2). The eight variables with the heaviest load in the three components were used in CAN–DISC. CANDISC showed that 98 % of total variation was explained by 3 canonical functions and 4 variables supplied the most information for discriminating among species. The variables that loaded most heavily were stipe scale length (16), basal pinna length (6), number of pinna pairs (5), and frond length (1) (Table 3). Mahalanobis distances (Md) supported the differences between species pairs (Md = 3.2765.23 , 0.003–p–0.0001), except between D. patula and D. rosea (Md = 1.32, p > 0.25)
Both similarity analyses showed the existence of two groups (matrixes available upon request to the author of correspondence). In the first analysis, the phenogram with 98 characters gave a cut–off point of 0.53 for the species of the complex (not shown, but available upon request to the author of correspondence). In the second analysis, the phenogram with only 84 characters showed fewer divisions, and the cut–off point for the species of the complex was 0.60 (figure 1). The comparative species, Dryopteris maxonii and D. wallichiana, differed from the D. patula complex and had similarity levels of less than 0.30 in both phenograms in comparison with species of the complex. Individuals of D. rossii were grouped with a similarity of 0.9 and were segregated because of the lustrous, oval–shaped rhizome scales, the light yellow to reddish–chestnut colored stipe with bicolored and lustrous scales, the rachis with pale scales and deltate blade, and the combination of glands and scales on the costae. Several individuals of D. rossii were grouped with a similarity of 1.0 although individuals came from different locations.

Individuals of Dryopteris cinnamomea formed another group far apart from individuals of D. rosea. The D. cinnamomea individuals were grouped by having scales on the rhizome with entire to subentire margin and lanceolate blade. Individuals of D. patula were grouped by the presence of the dentate margin of the rhizome and stipe scales. Individuals of D. rosea were grouped together because of their pinkish to reddish–brown stipe and rachis. As in D. rossii, there were several groups with maximum similarity values that came from different locations. In the case of D. cinnamomea, a group of 16 individuals came from 5 different locations (C42, C43, C47 from the Estado de México; C13, C14, C18 from the Distrito Federal; C27, C28 from Querétaro; C1, C2, C34, C31, C32 from Michoacán; C19, C20 from Hidalgo, and C9 from Veracruz).
Discussion
Statistical analyses. CANDISC showed that the number of pinna pairs, basal pinna length, and stipe scale length characters allow segregation of most species of the complex. For example, Dryopteris patula has the greatest number of pinna (mode = 16 ± 3). Pinnae are fewer in the other three taxa of the complex, D. cinnamomea (14 ± 3), D. rosea (14 ± 3) and D. rossii (14 ± 2). D. wallichiana has significantly more pinnae (mode = 31 ± 4) than all the other species and can thus be distinguished from the rest by sight alone. D. rossii has the longest basal pinna (mean = 13.0 ± 4.3 cm), and D. wallichiana has the shortest (7.2 ± 1.8 cm). For the third character, stipe scale length, D. rossii has the smallest scales (mean = 4.1 ± 1.0 mm), D. patula (12.8 ± 4.0 mm) and D. wallichiana (14.7 ± 2.2 mm) the largest scales, and D. cinnamomea (8.0 ± 3.0 mm) and D. rosea (8.6 ± 3.0 mm) have scales of intermediate size. The species with the largest fronds is D. patula (53.4 ± 16.8 cm); however in the other species of the complex frond size varies too much, overlapping between species, and making this character uninformative within the complex. D. patula could not be separated from D. rosea based exclusively on these quantitative characters. Moreover, Lee et al. (2006) have found that blade and stipe lengths, size and shape of scales on the rachis, and indusia size allowed them to delimit seven species in the
Dryopteris varia complex. The number of veins and the size of the blade are informative attributes of frond morphology that can distinguish among other Dryopteris species during frond development (Sato and Tsuyuzaki, 1988; Sato 1989).
Multivariate analyses can allow recognition of groups of species or populations and can detect characters that serve for their separation (Johnson, 2000), as shown in the results of the present study, where Dryopteris rossii and D. wallichiana could be distinguished. Although the characters indicated by the canonical discriminant analysis had not been considered important for the circumscription of the taxa studied previously, when combined with the qualitative characters, they enabled the segregation of all species within the D. patula complex.
Cluster analysis. In the phenogram, the first group contained the species of the Dryopteris patula complex distinct from the two species used for comparative purposes (figure 1). The four species of the complex are clearly distinct from one another. Dryopteris rosea individuals are grouped apart from D. cinnamomea individuals, thus our results do not support Mickel and Smith (2004) inclusion of the D. rosea as synonym of D. cinnamomea.
The grouping pattern of individuals reveals that some individuals are more similar between localities than within them. These results show that morphological variation did not always agree with geographic distribution, as has been found in other taxa (Terrazas and Wendt, 1995; Martínez–Cabrera, 2002; Aguilar–Morales, 2004). Inclusion of type specimens in analysis was highly valuable because if a set of characters distinguished species it can be assigned to a taxon. Similar taxonomic considerations arose for the species of the Isoëtes karstenii complex (Small and Hickey, 2001), which is why it is recommended to include the type specimens in multivariate analysis.
Mickel and Beitel (1988), Moran (1995), and Riba and Pérez–García (1999) considered the gland density on the blade, the size of scales on the rhizome and stipe, and the axes color as diagnostic characters for the species of the Dryopteris patula complex. Here we confirm that the size of the stipe scales was informative in both analyses to separate the species of the complex. In D. cinnamomea and D. rosea, the scales of the rhizome are of similar size (8 mm), whereas D. rossii has smaller scales (4 mm). Moreover, the pinkish to reddish–brown stipe segregates D. rosea in the phenogram. However, gland density on the blade was not included in the analyses because this character showed a continuous intraspecific variation. For example, in D. cinnamomea the C5 individual has 13 glands on the basal, 10 on the middle, and 7 on the apical blade, while the C14 individual presented 17 glands on the basal, 13 on the middle, and 20 toward the apical part. Hernández et al. (2006) found some anatomical characters that support the species recognition. For example, there are crystals on the periphery of sclereid nests in the rhizome of D. rossii, while intercellular spaces in the sclereid nests distinguished D. patula.
With respect to the characters that delimit each species of the Dryopteris patula complex in the cluster analysis, qualitative features such as color, shape, margin, and indument of the organs studied, contributed the most. This higher contribution is similar to what was reported for other species complexes (Speer and Hilu, 1999). In agreement with Mickel and Beitel (1988), D. rossii is distinguished from the other species of the complex by having a deltate blade. In addition, we propose five additional characters to support its distinctiveness: the ovate and lustrous scales in the rhizome, the light yellow to reddish–chestnut colored stipe with bicolorous and lustrous scales towards the base, pale scales in the rachis, and the combination of glands and scales on the costae. The characters that distinguish the individuals of D. cinnamomea are the scales of the rhizome with entire to subentire margin and the lanceolate blades. In D. rosea, the distinguishing characters are some features of the stipe: its length of 6.5–29 cm and its pinkish to reddish–brown color with scales scattered along its length. Dryopteris patula individuals are distinguished by scales with dentate margin in the rhizome and stipe. Moreover, D. patula includes the largest plants, with frond length of 29–76 cm; the scales of the stipe are larger with a length of 6.4–18 mm, with dentate margins, and the number of pinna pairs (16–26) is higher than in the other species of the complex. However, these differences need to be supported by a larger sampling including all the species distribution areas, because only eight specimens were included in the analyses due to frond fragmentation commonly found in herbarium sheets. D. knoblochii according to Mickel and Smith (2004) differs from D. rossii in the concolorous scale base that lacks blackened tips, while with D. cinnamomea mainly differs by having thicker blades and smaller indusia. However, the indusium is similar in size to that of D. rossii. Other morphological characters are similar with D. patula and D. rosea studied here. The evaluation of D. knoblochii needs to be studied morphometrically.
The characters studied by the multivariate analyses revealed a unique combination of morphological features that allows us to distinguish each species of the Dryopteris patula complex. However, we recommend to include further D. knoblochii and also, to consider an independent source of data such as molecular markers.
Taxonomic treatment
Key to the species of Dryopteris patula complex
1. Blade deltate, rachis with glands and scales, rhizome scales ovate, stipe scales bicolorous, basal pinna large (mean = 13.0 ± 4.3 cm)...........................................D. rossii
1. Blade lanceolate to deltate to lanceolate, rachis with glands only, rhizome scales ovate lanceolate, stipe scales concolorous, basal pinna small (mean < 12.0 cm)............................2
2. Margin of the rhizome scales toothed to crisped, basal pinna (9.0 ± 3.3 cm)..........................................D. patula
2. Margin of the rhizome scales entire or subentire, basal mean < 8 cm.................................................................... 3
3. Blade lanceolate, stipe and rachis stramineous..................................................................... D. cinnamomea
3. Blade deltate to lanceolate, stipe and rachis pinkish to reddish–brown..............................................D. rosea
Dryopteris cinnamomea (Cav.) C. Chr., Amer. Fern J. 1:95. 1911. Figures 2A, 3.
Tectaria cinnamomea Cav., Descr. Pl. 252. 1801. Type. México. [Mexico:] Chalma, Née s.n. (MA, frag. S).
Nephrodium mexicanum C. Presl, Reliq. Haenk. 38. 1825. Dryopteris mexicana (C. Presl) C. Chr., Kongel. Danske Vidensk. Selsk. Skr., Naturvidensk Math. Afd., ser. 7, 10: 16. 1913. Type. Mexico. Haenke s.n. (PRC; photo NY).
Aspidium athyrioides M. Martens & Galeotti, Mém. Foug. Mexique 67, pl. 18. 1842. Dryopteris athyrioides (M. Martens & Galeotti) Kuntze, Revis. Gen. Pl. 2: 811. 1891. Type. Mexico. [Hidalgo:] "Real–del–Monte," 8000–8500 ft, Galeotti 6425 (P, photo US, frag. NY; isotype K).
Polystichum cystopteroides Nees, Linnaea 19: 685. 1847. Type. Mexico. Aschenborn 192 (B?).
Polypodium glanduliferum Liebm., Mexic. Bregn. 206 (reprint 54). 1849. Dryopteris glandulifera (Liebm.) C. Chr., Index Filic. 267. 1905. Syntypes. Mexico. Oaxaca: Inter Comaltepec et Trapiche de la Concepción, Liebmann s.n. [Pl. Mex. 2395, Fl. Mex. 196, 197] (C; isotype K).
Lastrea indecora Liebm., Mex. Bregn. 272 (reprint 120). 1849. Dryopteris indecora (Liebm.) C. Chr., Index Filic. 272. 1905. Type. Mexico. Oaxaca: Yavesia, Liebmann s.n. [Pl. Mex. 2417, Fl. Mex. 465] (C, frag. US).
Aspidium agatolepis Fée, Mém. Foug. 8: 106. 1857. Type. Mexico. S. Agostín Schaffner 309b (RB, cited by Win–disch, 1982: 57).
Aspidium flaccidum E. Fourn., Bull. Soc. Bot. France 27: 328. 1880, hom. Illeg., non Blume, 1828. Nephrodium fournieri Baker, Ann. Bot. (London) 5: 317. 1891, non Baker, op cit. 328 (photo P!) TC*. Dryopteris fournieri (Baker) C. Chr., Index Filic. 266. 1905. Type. Mexico. San Luis Potosí: Schaffner 85 (photo P!, NY!, frag. NY).
Rhizome erect, scales reddish–brown concolorous, ovate–lanceolate with margin entire or subentire, cross sections with sclereid nest and without crystals on the periphery, stipe 2.5–18 (mean = 8.4 ± 3.5) cm long, stramineous, with glands and scales 3–15 (8.0 ± 3.0) mm long, concolorous, near base, with margin entire and acuminate tips; rachis stramineous, densely glandular; blade 8–41 (18.0 ± 7.3) cm long, 3–16 (7.7 ± 3.1) cm wide, bipinnate–pinnatifid, lanceolate, with glands both adaxial and abaxial side and the veins; number of pinna pairs 8–20 (14.0 ± 2.6); basal pinna 2–12 (4.5 ± 2.0) cm long, 1.3–5 (2.6 ± 0.7) cm wide; costae with glands; indusia flat, reniform, with glands, grayish–brown; spore monolete 31–43 x 27–36 μm.
Examined material: MÉXICO. DISTRITO FEDERAL: Deleg. Milpa Alta, M.E. Cisneros CIPEM 4/2 (ENCB) C18; Deleg. Coyoacán, J. Rzedowski 963 (ENCB) C13, C14; De–leg. Tláhuac, M.L. Arreguin 656 (ENCB) C21; ESTADO DE MÉXICO: Mpio. Amecameca, M.E. Cisneros CABORP 3/8 (ENCB) C15, C16, A.C. Luque 24 (ENCB) C17, V. Hernández H. y E. Martínez V. 371 (CHAPA) C48, V. Hernández H. y E. Martínez V. 372 (CHAPA) C38, C39, V. Hernández H. y E. Martínez V. 373 (CHAPA) C47, V. Hernández H. y E. Martínez V. 374 (CHAPA) C45, V. Hernández H. y E. Martínez V. 378 (CHAPA) C40, C41, V. Hernández H. y E. Martínez V. 380 (CHAPA) C42, C43, V. Hernández H. y E. Martínez V. 381 (CHAPA) C44, V. Hernández H. y E. Martínez V. 382 (CHAPA) C46; GUANAJUATO: Mpio. Guanajuato, S. Gutiérrez 1 (IEB) C26, Cano–Mares y A. Estrada 130 (MEXU) C3, C4; Mpio. San Luis de la Paz, J. Rzedowski s.n. (IEB) C35; Mpio. Victoria, Carranza, Zamudio y Pérez 4364 (MEXU) C7; HIDALGO: El Chico, M.L. Arreguin 647 (ENCB) C19, C20; MICHOACÁN: Mpio. Acuitzio, H. Díaz–Barriga 5074 (IEB) C25; Mpio. Contepec, J. Rzedowski 51844 (MEXU) C6; Mpio. Morelia, J. Rzedowski 42125 (ENCB) C22, C31, C32; Mpio. Paracho, E. García y E. Pérez 3264 (MEXU) C1, C2; Mpio. Zinapécuaro, Almazan et al. 820 (IEB) C23, C24, J. Rzedowski 46065 (IEB) C33, C34; NAYARIT: Mpio. Nayar, G. Flores F. et al. 2203 (MEXU) C8; OAXACA: Dist. Villa Alta, J.T. Mickel 865 (ENCB) C10; Dist. Sola de Vega, J.T. Mickel 1655 (ENCB) C11; QUERÉTARO: Mpio. Amealco, J. Rzedowski 51106 (MEXU) C5, (IEB) C27, C28; Mpio. Peñamiler, S. Zamudio 5981 (IEB) C29, C30; VERACRUZ: Mpio. Jalacingo, F. Ventura A. 14524 (ENCB) C12; Mpio. Jilotepec, D.S. Barrington 429 (MEXU) C9; Mpio. Tlacolulán, V. Hernández H. et al. 332 (CHAPA) C36, V. Hernández H. et al. 345 (CHAPA) C37. In bold identification of the specimens used in figure 1.
Dryopteris patula (Sw.) Underw. Our native ferns ed. 4, 117. 1893. Figures 2B, 3
Aspidiumpatulum Sw., Kongl. Vetensk. Acad. Handl. 1817: 64. 1817. Type. Brazil. Minas Gerais, Freyreiss s.n. (photo S!) TP*.
Aspidium paupertinum Kunze, Linnaea 18: 345. 1844. Aspidium mexicanum C. Presl var. obtusilobum Kunze ex Mett., Abh. Senckeberg, Naturf. Ges. 2: 349. 1858. Type. Mexico? 'Reg. temp. (Herb. Roem., propr) Coll. No. 42".
Aspidium apertum Fée, Mém. Foug. 8: 106. 1857. Dryopteris aperta (Fée) C. Chr. Index Filic. 252. 1905. Syntypes. Mexico. [Veracruz:] Huatusco, Schaffner 73 (P?); [Morelos:] Cuernavaca, Craveri 73b (P).
Dryopteris simplicior Mickel & Beitel, Mem. New York Bot. Gard. 46:166. 1988. Type. Mexico. Oaxaca: Distrito Putla, 3 km N of Putla, Mickel 3977 (NY).
Rhizome erect, scales reddish–brown concolorous, ovate–lanceolate, the margin toothed to crisped, cross sections with sclereid nest with intercellular spaces and without crystals on the periphery, stipe 10–33 (mean = 17.9 ± 7.6) cm long, stramineous, glandular throughout, scaly near the base, the scales 6.4–18 (12.8 ± 4.0) mm long, concolorous, margin toothed and long twisted tips; rachis stramineous with glands; blade 20–51 (35.6 ± 11.0) cm long, 8–24 (16.1 ± 5.7) cm wide, tripinnate–pinnatifid, deltate to lanceolate, glandular in the groove adaxially; number of pinna pairs 1626 (18.9 ( 3.4); basal pinna 5–15 (9.0 ± 3.3) cm long 2.7–7 (4.3 ± 1.7) cm wide; indusia flat, reniform or cordate, with glands, brown to tan; spores monolete 24–47 × 20–36 μm.
Examined material: MÉXICO. OAXACA: Dto. Putla, E. Solano C. 501 (CHAPA) P2; VERACRUZ: Mpio. Jilotepec, V. Hernández H. y p.F. Franco H. 461 (CHAPA) P7, V. Hernández H. y p.F. Franco H. 469 (CHAPA) P8; Mpio. Las Vigas de Ramírez, V. Hernández H. et al. 325 (CHAPA) P6; Mpio. Tlacolulán, V. Hernández H. et al. 330 (CHAPA) P5, V. Hernández H. et al. 348 (CHAPA) P3; P4. In bold identification of the specimens used in figure 1.
Dryopteris rosea (E. Four.) Mickel & Beitel, Mem. N.Y. Bot. Gard. 46:165. 1988. Figures 2C, 3
Aspidium roseum E. Four., Mexic. Pl. 1:97. 1872. Lectotype. México. Orizaba, Müller 4bis (P?); isotype (photo NY!) TR*.
Rhizome erect, scales reddish–brown concolorous, ovate–lanceolate with margin entire, cross sections present sclereid nest without crystals on the periphery, stipe 6.5–29 (mean = 13.8 ± 6.3) cm long; pink to reddish–brown, with glands and scales of 4–15 (8.6 ± 3.0) mm, concolorous, scales sparse along stipe, margin entire with long twisted tips; rachis pink to reddish–brown, densely glandular; blade 7.5–50 (27.2 ± 11.0) cm long, 4–25 (12.5 ± 5.6) cm wide, bipinnate pinnatifid, deltate lanceolate; few glands in both adaxial and abaxial side, only glands in groove of the costule; number of pinna pairs 10–20 (16 ± 2.6); basal pinna 2–19 (7.1 ± 4.0) cm long, 1.3–9 (3.8 ± 1.7) cm wide; indusia flat, reniform or cordate (horseshoe–shaped), with glands, brown to tan; spore monolete 36–46 × 27–34 μm.
Examined material: MÉXICO. ESTADO DE MÉXICO: Mpio. Amecameca, V. Hernández H. y E. Martínez V. 365 (CHAPA) R26, V. Hernández H. y E. Martínez V. 366 (CHAPA) R27, V. Hernández H. y E. Martínez V. 367 (CHAPA) R25, V. Hernández H. y E. Martínez V. 377 (CHAPA) R24; GUERRERO: Mpio. Chichihualco, F. Lorea 3914 (IEB) R1, R2, F. Lorea 3131 (IEB) R5, R8, R11, F. Lorea 2496 (IEB) R7, J. Rzedowski 18523 (ENCB) R12, R13; Mpio. Tlacotepec, F. Lorea 2098 (IEB) R3, R4; JALISCO: Mpio. Autlán de Navarro, R. Cuevas et al. 4333 (CHAPA) R14; Mpio. Autlán de Navarro R. Cuevas et al. 4295 (CHAPA) R15; MICHOACÁN: Mpio. Ucareo, H. Díaz–Barriga 4239 (IEB) R6; OAXACA: Dist. Central, J.T. Mickel 1169 (ENCB) R10; Dto. Ixtlán, J.T. Mickel et al. 4428a (MEXU) R9; VERACRUZ: Mpio. Las Vigas de Ramírez, V. Hernández H. et al. 324 (CHAPA) R17, V. Hernández H. et al. 327 (CHAPA) R18, V. Hernández H. et al. 328 (CHAPA) R19, R20, V. Hernández H. et al. 339 (CHAPA) R23, V. Hernández H. et al. 342 (CHAPA) R21, V. Hernández H. et al. 343 (CHAPA) R22, V. Hernández H. et al. 349 (CHAPA) R16. In bold identification of the specimens used in figure 1.
Dryopteris rossii C. Chr. Mem. Acad. Nac. Ci. "Antonio Alzate" 32:178. 1912. Figures 2D, 3
Dryopteris patula (Sw.) Underw. var. rossii (C. Chr.) C. Chr., Kongel. Danske Vidensk. Selk. Skr., Naturvidensk. Math. Afd., ser. 7, 10: 20. 1913. Lectotype (chosen by Christensen, 1913: 22). Mexico. Morelos. Cuernavaca, Santa Maria, Ross 279 (M; isolectotype, photo P!) TI*.
Rhizome erect, scales blackish–brown, concolorous, ovate with margin entire; cross sections present sclereid nest with crystals on the periphery, stipe 8–41 (mean = 21.7 ± 7.0) cm long, stramineous to reddish–brown to base, with glands and scales of 2.3–6.5 (4.1 ± 1.0) mm long, bicolorous, scales sparse along stipe, margin entire with lustrous dark tips and pale brown base; blade 15–43 (27.2 ± 7.0) cm long, 5.2–37 (16.4 ± 5.4) cm wide, tripinnate pinnatifid otherwise mostly bipinnate–pinnatifid, deltate, densely glandular in both ad–axial and abaxial side and the veins; number of pinna pairs 10–19 (14.2 ± 2.0); basal pinna 4.2–24 (13.0 ± 4.3) cm long, 2–13 (6.3 ± 2.5) cm wide; indusia flat, reniform or cordate, with glands, brown to tan; spore monolete 33–43 × 24–32 μm.
Examined material: MÉXICO. ESTADO DE MÉXICO: Mpio. Tejupilco, V. Hernández H.y E. Martínez V. 391 (CHAPA) I45, I46, V. Hernández H.y E. Martínez V. 393 (CHAPA) I44, V. Hernández H. y E. Martínez V. 397 (CHAPA) I38, I39, V. Hernández H.y E. Martínez V. 396 (CHAPA) I40, I41, I42, I43, V. Hernández H.y E. Martínez V. 399 (CHAPA) I37, V. Hernández H.y E. Martínez V. 400 (CHAPA) I36, V. Hernández H.y E. Martínez V. 402 (CHAPA) I35, V. Hernández H.y E. Martínez V. 403 (CHAPA) I34, A. Flores et al. 973 (CHAPA) I51; Mpio. Valle de Bravo, H. Sánchez–Mejorada 818 (ENCB) I9, I10; GUERRERO: Mpio. Alcozauza, F. Lorea 2771 (ENCB) I13; Mpio. Atlixtac, F. Lorea 2741 (ENCB) I17; Mpio. Buena Vista de Cuellas, F. Lorea 4681 (IEB) I1; Mpio. Chilpancingo, J. Rzedowski 22725 (ENCB) I20; Mpio. La Unión, F. Lorea 2549 (ENCB) I14, (MEXU) I4; Mpio. San Luis Acatlán, F. Lorea 2839 (ENCB) I12; Mpio. Taxco, F. Lorea 3225 (ENCB) I15, I18, (IEB) I2, F. Lorea 4454 (ENCB) I19; Mpio. Tixtla, F. Lorea 1428 (ENCB) F. Lorea 1316 (ENCB) I16; JALISCO: Mpio. Ayutla, W.R. Anderson 12761 (CHAPA) I50; Mpio. Tecalitlán, C. Feddema 2210 (ENCB) I22; Mpio. Tlalpa de Allende, F. Boutin y F. Brandt 2599 (ENCB) I21; MICHOACÁN: Mpio. Erongaricuaro, J.M. Escobedo 1252 (ENCB) I27; Mpio. Morelia, J. Santos M. 1086 (ENCB) I23, J. Rzedowski 42126 (ENCB) I24, J. Rzedowski 45312 (ENCB) I25; Mpio. Pátzcuaro, V. Hernández H. y S. Zamudio 452 (CHAPA) I47, V. Hernández H. y S. Zamudio 455 (CHAPA) I48, V. Hernández H. y S. Zamudio 459 (CHAPA) I49; Mpio. Quiroga, K. Roe y E. Roe 2047 (ENCB) I29; Mpio. Santa Clara del Cobre, E. Pérez–Cálix 318 (IEB) I3; Mpio. Zacapu, J. Rzedowski 45880 (ENCB) I26; Mpio. Zinapécuaro, H. Díaz–Barriga 6791 (MEXU) I5; Mpio. Zitácuaro, J. Rzedowski 18346 (ENCB) I28; NAYARIT: Mpio. Acaponeta, O. Tellez H. y A. Salinas 12033 (ENCB) I32; Mpio. Compostela, R. McVaugh 16452 (MEXU) I6; Mpio. Compostela, C. Feddema 2661 (ENCB) I33; Mpio. Tepic, R.E. Gonzalez et al. 802 (MEXU) I7, D.E. Breedlove 45180 (ENCB) I30, I31; ZACATECAS: Mpio. Juchipila, J.J. Balleza y M. Adame 11387 (MEXU) I8. In bold identification of the specimens used in figure 1.
Acknowledgements
The senior author thanks Consejo Nacional de Ciencia y Tecnología for a scholarship (162688). We are grateful to Colegio de Postgraduados for logistical support, to René Hernández–Gómez, Pedro Franco H., Enrique Martínez v., Valentín J. Reyes H., and Cesáreo Catalán–Hevarístico for their assistance with field work, to Jorge Vargas for the illustrations, to Klaus Mehltreter and Michael Sundue for critical comments and linguistic improvement to the manuscript, and to two anonymous reviewers.
Literature cited
Aguilar–Morales I. 2004. Variación morfológica de Stenocereus dumortieri (Cactaceae). Tesis licenciatura, Facultad de Ciencias, Universidad Nacional Autónoma de México, México, D.F. 74 pp. [ Links ]
Crisci J.V. and López–Armengol M.F. 1983. Introducción a la teoría y práctica de la taxonomía numérica. Monografía No. 26, serie Biología. Organización de Estados Americanos. Washington, D.C. [ Links ]
Hernández V., Terrazas T. and Angeles G. 2006. Anatomía de seis especies de helechos del género Dryopteris (Dryopteridaceae) de México. Revista de Biología Tropical 54:1157–1169. [ Links ]
Johnson D.E. 2000. Métodos multivariados aplicados al análisis de datos. International Thomson. México. [ Links ]
Lee S.J., Kim Y.D., Suh Y., Lee S.K. and Park C.W. 2006. Morphological and chromosomal variation of the Dryopteris varia (L.) Kuntze complex (Dryopteridaceae) in Korea. Plant Systematics and Evolution 262:37–52. [ Links ]
Martínez–Cabrera D. 2002. Variación morfométrica de dos especies de encinos rojos: Quercus sartorii Leibmann y Quercus xalapensis Humblodt & Bonpland (Fagaceae). Tesis Maestría, Programa en Botánica, Colegio de Postgraduados, Estado de México, 101 pp. [ Links ]
Mayer M.S. and Mesler M.R. 1993. Morphometric evidence of hybrid swarms in mixed populations of polystichum munitum and p. imbricans (Dryopteridaceae). Systematic Botany 18:248–260. [ Links ]
Mickel J.T. and Beitel J.M. 1988. Pteridophyte Flora of Oaxaca, Mexico. Memoirs of the New York Botanical Garden vol 46. The New York Botanical Garden Press. New York. [ Links ]
Mickel J.T. and Smith A.R. 2004. The Pteridophytes of Mexico. Memoirs of the New York Botanical Garden vol. 88. The New York Botanical Garden Press. New York. [ Links ]
Moran R.C. 1995. Dryopteris Adans., nom. cons. En: Davidse G., Sousa M. and Knapp S. Eds. Flora Mesoamericana: Volumen 1: Psilotaceae a Salviniaceae, pp. 212–214, Universidad Nacional Autónoma de México, Missouri Botanical Garden and The Natural History Museum (London). México. [ Links ]
Paris C.A.and Windham M.D. 1988. A biosystematic investigation of the Adiantum pedatum complex in eastern North America. Systematic Botany 13:240–255. [ Links ]
Pérez–García B. and Riba R. 1990. Glosario para pteridophyta (Helechos y plantas afines). Consejo Nacional de Flora de México A.C. México, D.F. [ Links ]
Riba R. and Pérez–García B. 1999. Pteridofitas: familia Dryopteridaceae. Flora de México vol 6, no 4. Consejo Nacional de la Flora de México A.C. México, D.F. [ Links ]
SAS, Institute Inc. 1989. SAS procedure guide. Release 6.03. SAS Institute Inc. Cary, North Carolina. [ Links ]
Sato T. and Tsuyuzaki S. 1988. Quantitative comparison of foliage development among Dryopteris monticola, D. tokyoensis and a putative hybrid, D. kominatoensis in northern Japan. Journal of plant Research 101:267–280. [ Links ]
Sato T. 1989. A Quantitative estimation of sequential foliage development and fertility in Dryopteris crassirhizoma. Journal of plant Research 102:445–455. [ Links ]
Small R.L. and Hickey R.J. 2001. Systematics of the northern Andean Isoëtes karstenii complex. American Fern Journal 91:4169. [ Links ]
Speer W.D. and Hilu K.W. 1999. Relationships between two in–fraspecific taxa of Pteridium aquilinum (Dennstaedtiaceae). I. Morphological evidence. Systematic Botany 23:305–312. [ Links ]
Terrazas T. and Wendt T. 1995. Systematic wood anatomy of the genus Tapirira Aublet (Anacardiaceae) – a numerial approach. Brittonia 47:109–129. [ Links ]
Waterway M.J. 1986. A reevaluation of Lycopodium porophilum and its relationship to L. lucidulum (Lycopodiaceae). Systematic Botany 11:263–276. [ Links ]














