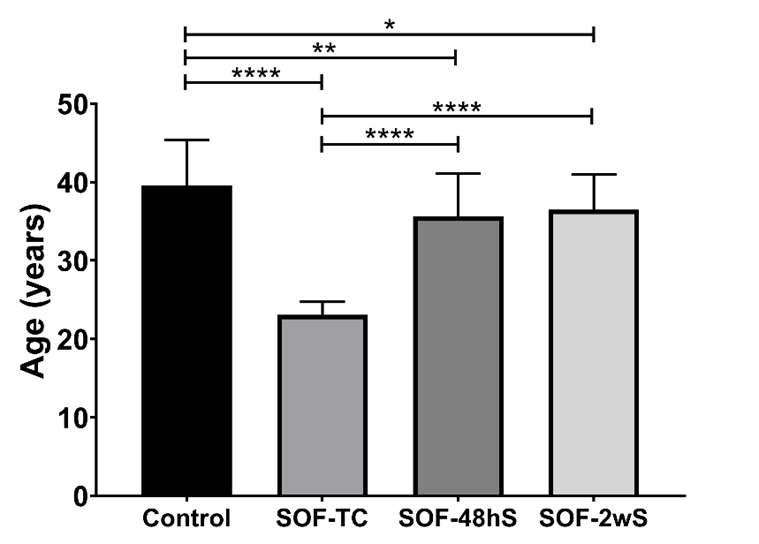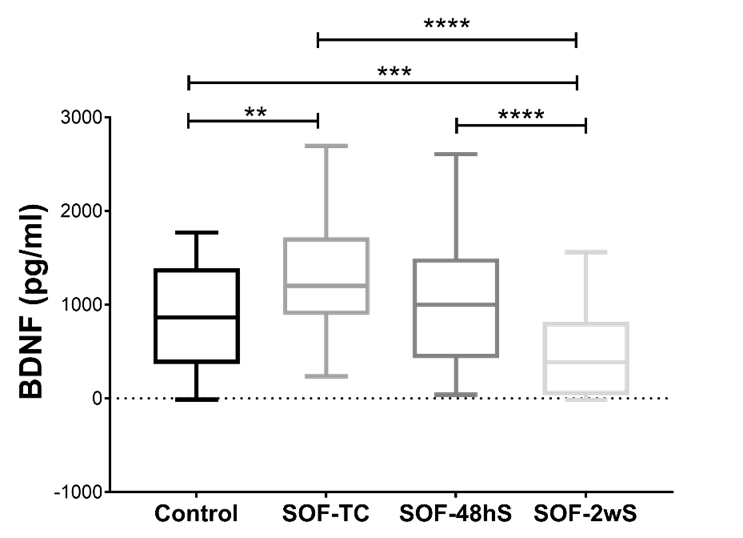Introduction
The military units aim train to qualified troops with high efficiency, not only requiring military skills, equipment, and adequate tactics, but also adequate physical and mental preparation soldiers.1
Soldiers often do dangerous and stressful work; physically demanding activities include carrying and transporting heavy materials, digging, and shoveling, as well as prolonged physical activity with additional loads of combat equipment weighing between 25 and 65 kg. In addition to being subjected to multiple stressors due to their functions, various factors can affect the ability to work, such as lack of sleep, continuous physical activity, and mood disorders.1,2
Military training is physically and mentally demanding, it can have a negative impact on physical and mental efficiency,3 so having biomarkers that reflect the severity of mental stress is necessary.4
Using biomarkers may be advantageous, as truthfulness in self-reporting is a concern, particularly for active-duty military personnel who may experience significant adverse consequences (e.g., dismissal from active service, not following through with treatment recommendations) by receiving a mental health diagnosis, developing accurate ways to diagnose beyond clinical interviews may be essential.5
Brain-derived neurotrophic factor (BDNF) is a neurotrophic factor that is highly expressed in the hippocampus. It serves diverse functions such as neuronal survival, midbrain dopaminergic and cholinergic neuron maintenance, and synaptic plasticity. It also plays a role in memory, learning, appetite, and sleep processes.6-9 Alterations in BDNF levels, as seen in hippocampus or peripheral serum tests, are related to neuropsychiatric disorders such as dementia, depression, anxiety, schizophrenia, and bipolar disorder. It has also been well documented that beneficial BDNF serum levels can be re-established for individuals after treatment with antidepressants, mood stabilizers and electroconvulsive therapy, aiding in recovery.6,8,10
Presently, the neuro-molecular approach emphasizes the use of biomarkers related to psychiatric disorder diagnosis.11 For example, some BDNF research has focused on the correlation between major depressive disorder and suicide risk. It has been documented that BDNF levels decrease in patients with major depressive disorder, and that low levels of blood platelets occur in recently suicidal patients.12-14
BDNF levels in serum and the brain have demonstrated levels decrease with age and that lower serum BDNF levels are associated with lower spatial memory performance.15
The current study examined BDNF serum levels in four Mexican military samples with special training and without training. It was hypothesized that BDNF levels would decrease after operational military stress exposure; therefore, the control group would have higher levels of BDNF than the test groups.
Materials and methods
This study was developed in affiliation with the Military Health Graduate School and ethical approval was granted by the Institutional Human Research Ethical Committee of the School. Participants were male active-duty members of the Mexican Army and Air Force who did not have current or previous diagnoses of mental health disorders, substance-related disorders, or chronic degenerative disease. Individuals who had or were presently undergoing psychopharmacological treatment were also excluded. Participants were selected, received no compensation for participation, the participation was voluntary, and groups were naturally occurring.
Overall, 132 participants aged 20 to 48 consented to participate between July and December 2017. This population was divided into four sample groups, with the first group acting as a control group as it was comprised of administrative personnel (n=31) who were not exposed to any operator training during this study. The three remaining samples were all comprised of Special Operations Forces members (SOF) and had varying levels of operational stress exposure. The first test group (n=34) consisted of SOF basic training course (SOF-TC) participants who were exposed to stressful combat simulations. The second test group (n=34) was exposed to 48 hours of operational stress (SOF-48hS) and the third test group (n=33) was exposed to two-weeks of operational stress (SOF-2wS). The SOF-48hS and SOF-2wS groups consisted of two different military units of which comprised of the most qualified members of the Mexican SOF community with specialized tactical training.
During the study, operational stress was not controlled by the authors but instead dictated by the SOF. Possible operational stress scenarios included ambushes and surprise attacks (i.e., constant vigilance); confrontations with drug cartels leading to armed confrontations, foot and vehicle pursuits, and sleep and food deprivation. No significant injuries occurred while undergoing these military-directed trainings.
At the end of the operational training, the mini-interview and CAPS were applied; participants consented to having 5 mL of blood taken via venous puncture. Blood samples were collected by military nurses in sample tubes that were then centrifugated to 3500 rpm for 20 minutes. After that, the sample was stored at -112 °F (-80 °C) until the analysis was conducted. Molecular biology specialist conducted all the blood sample analyses.
Levels of BDNF in the blood samples were determined by conducting a Sandwich enzyme-linked immunosorbent assay (ELISA) which is considered a gold-standard in identifying protein species 16. Specifically, the ELISA HUMAN BDNF PicoKine EK308 kit was utilized according to the manufacturer’s instructions (Booster Biological Technology, CA. USA) and the 96 wells plate was read on a HIDEX Sense 425-301, EU. The specifications were 15 pg/ml for BDNF.
Age, time in service, and BDNF levels did not show a normal distribution by the Kolmogorov-Smirnov test. Differences in age, time in service, and BDNF levels were evaluated by the Kruskal-Wallis test and post hoc Dunn’s. To analyze the relationships between the BDNF serum levels and demographics, the Spearman moment correlation coefficient was utilized. The statistical analyses were completed using SPSS version 25 software with p < 0.05 indicating statistical significance.
Results
The mean age of the military population was 33.42 (SD=7.91) years old. The average age of each sample was as follows: 39.59 years (SD=5.8) for the control group, 23.09 years (SD=1.66) for SOF-TC, 35.59 years (SD=5.52) for SOF-48hS, and 36.47 years (SD=4.51) for the SOF-2wS sample (Figure 1). The largest age difference existed between the SOF-TC and control samples.
Age in Control, SOF-TC (Special operations forces on training course), SOF-48hS (Special operations forces-stressed by 48 h), and SOF-2wS (Special operations forces stressed for two weeks). mean±SD, ***p<0.0001. ANOVA test and post-hoc Tukey´s.
The time of active service for each sample group was as follows: the control group served 18.13 (SD=6.03 years), the SOF-TC served 3.44 (SD=1.62 years), the SOF-48hC served 16.86 (SD=5.29 years) and the SOF-2wS served 17.13 (SD=4.80 years). The time of active service was statistically significant between the control and SOF-TC samples.
In the mini-interview no alterations were detected. The CAPS findings showed that a participant of the control group and 2 participants of the SOF-2wS group, met criteria for PTSD. In contrast, no participant from the SOF-TC or SOF-48hS samples reported PTSD symptoms. The participants who screened positively for a PTSD diagnosis were between the ages of 34 and 38 years old.
A low inverse correlation was found between age and BDNF levels Rho Spearman de -0.245, p=0.001.
For the BDNF levels, the lowest mean levels were found in the SOF-2wS group at a rate of 487.8 pg/ml (SD=469.3), while the highest mean levels were found in the SOF-TC group with 1310 pg/ml (SD = 576.7). The control group’s mean level was 886.2 pg/ml (SD=528.7) while the SOF-48hS sample demonstrated a mean level of 1039 pg/ml (SD=697.4). Significant differences in BDNF were found between the levels of the control group and the SOF-2wS, as well as between the control group and the SOF-TC samples’ levels (Figure 2).
Also, there are significant differences between SOF-TC vs SOF-2wS and SOF-48h vs SOF-2wS.
Low levels of BDNF have been reported related to multiple disorders and during aging, which are usually accompanied by moderate atrophy, reduced neuronal function and loss of synapses.16,17 This is consistent with our findings, observing a low inverse correlation between age and BDNF levels.
The BDNF is a neurotrophic highly related to mental disorders like Major Depressive Disorder and PTSD. The central role of BDNF is related to neuronal plasticity, neurogenesis, and response to brain insults.18-20 Study found 886.2 ± 528.7 pg/ml serum levels of healthy and control volunteers, similar results to those reported by other authors.8
The SOF-TC group was positively reinforced with high level Special Forces training. A significant increase (p=0.0013) of BDNF levels in this group was observed. This observation was expected as it has been well described in animal studies that adding a physical exercise component increases BDNF levels in the hippocampus and the cortex. Thus, improving synaptic plasticity and neurogenesis,19 also in the mature nervous system, BDNF is involved in activity-dependent synaptic plasticity and seems to protect neurons against different kinds of brain insult.21
In the group SOF-48hS a comparable increase in BDNF in the control group was found, indicating non-significant differences. There are controversial results on BNDF levels after exercise, most of them showed an increase of BNDF levels, but in some cases, especially in resistant training, at the beginning there is not significant changes in BDNF levels, on those cases the increase was observed after 10 weeks of training.22
While the SOF-2wS participants were under physiological and physical operative stress for two weeks, they showed a notable decrease in BDNF levels in comparison with all the other groups. Suzuki et al 2014, showed that after 3 weeks of military training, the BDNF levels decrease, but return to basal levels after 3 to 5 days.4 Although, low levels of BDNF are related to psychosocial stress on healthy volunteers; Suzuki et al showed a decrease in plasma BDNF concentration did not correlate with subjectively measured stress-VAS questionnaire answers, fatigue-VAS questionnaire answers, serum CPK, or sleep time.
Furthermore, on these group 2 participants showed signs of PTSD. Tural and colleagues (2018) reported similar levels in comparable PTSD groups.9
Despite this, the diagnostic process of PTSD remains a challenge due to various complicating factors such as heterogeneity in symptom presentation, stigma, and the possibility of malingering for financial gain in certain populations (e.g., motor vehicle accident survivors).23 Therefore, the psychiatric diagnosing of PTSD must take into consideration the cultural, social, economic, and historical context of the individual as well.24
In the literature, there have been differing results about the changes in BDNF levels on one’s PTSD symptoms, with the main variables being treatment history, psychiatric comorbidity, primary diagnosis, and temporal distance from trauma exposure.6 For example, Dell´Osso et al, in 2009 showed low levels of BDNF in PTSD patients without previous treatment or psychiatric comorbidity compared with healthy subjects. Additionally, another study found that a PTSD group had higher BDNF levels compared to healthy subjects, with the higher levels associated with more recent trauma (i.e., traumatic episode in the last year) as compared to more remote traumas (i.e., traumatic event occurring more than four years earlier.18,24,25
In remote trauma, BDNF and TRkB (receptor for the neurotrophies BDNF) signaling were correlated with clinical manifestation such as intrusive and incomplete memories of the distressing event, hyperarousal, fear memory formation and extinction, as well as restricted range of affect.26 These findings on remote trauma correspond with this study’s findings from the SOF-2wS group.
One of the limitations of the present study is that it only included male participants. Excluding female participants was not an intentional decision but a result of the specific population studied. As women worldwide are at an increased risk for developing PTSD symptoms due to being more frequently targeted for sexual violence (e.g., rape, sexual abuse), the inclusion of female Mexican Special Forces members would have strengthened these findings. For this study the type of exercise was not register. Thus, interpretations from these findings should be presented cautiously when being generalized beyond men.
Discussion
Low levels of BDNF have been reported related to multiple disorders and during aging, which are usually accompanied by moderate atrophy, reduced neuronal function and loss of synapses.17 This is consistent with our findings, observing a low inverse correlation between age and BDNF levels.
The BDNF is a neurotrophic highly related to mental disorders like Major Depressive Disorder and PTSD. The central role of BDNF is related to neuronal plasticity, neurogenesis, and response to brain insults (18-20). This study found 886.2±528.7 pg/ml serum levels of healthy and control volunteers, similar results to those reported by other authors.8
The SOF-TC group was positively reinforced with high level Special Forces training. A significant increase (p=0.0013) of BDNF levels in this group was observed. This observation was expected as it has been well described in animal studies that adding a physical exercise component increases BDNF levels in the hippocampus and the cortex. Thus, improving synaptic plasticity and neurogenesis,19 also in the mature nervous system, BDNF is involved in activity-dependent synaptic plasticity and seems to protect neurons against different kinds of brain insult.21
In the group SOF-48hS a comparable increase in BDNF to the control group was found, indicating non-significant differences. There are controversial results on BNDF levels after exercise, most of them showed an increase of BNDF levels, but in some cases, especially in resistant training, at the beginning there is not significant changes in BDNF levels, on those cases the increase was observed after 10 weeks of training.22
While the SOF-2wS participants were under physiological and physical, operative stress for two weeks, they showed a notable decrease in BDNF levels in comparison with all the other groups. Suzuki et al. in 2014 showed that after 3 weeks of military training, the BDNF levels decrease, but return to basal levels after 3 to 5 days.4 Although, low levels of BDNF are related to psychosocial stress on healthy volunteers; Suzuki et al showed a decrease in plasma BDNF concentration did not correlate with subjective measured stress-VAS questionnaire answers, fatigue-VAS questionnaire answers, serum CPK, or sleep time.
Furthermore, on these group 2 participants showed signs of PTSD. Tural and colleagues (2018) reported similar levels in comparable PTSD groups.9
Despite this, the diagnostic process of PTSD remains a challenge due to various complicating factors such as heterogeneity in symptom presentation, stigma, and the possibility of malingering for financial gain in certain populations (e.g., motor vehicle accident survivors).23 Therefore, the psychiatric diagnosing of PTSD must take into consideration the cultural, social, economic, and historical context of the individual as well.24
In the literature, there have been differing results about the changes in BDNF levels on one’s PTSD symptoms, with the main variation being treatment history, psychiatric comorbidity, primary diagnosis, and temporal distance from trauma exposure.6 For example, Dell´Osso et al. showed low levels of BDNF in PTSD patients without previous treatment or psychiatric comorbidity compared with healthy subjects. Additionally, another study found that a PTSD group had higher BDNF levels compared to healthy subjects, with the higher levels associated with more recent trauma (i.e., traumatic episode in the last year) as compared to more remote traumas (i.e., traumatic event occurring more than four years earlier.18-25
In remote trauma, BDNF and TRkB (receptor for the neurotrophies BDNF) signaling were correlated with clinical manifestation such as intrusive and incomplete memories of the distressing event, hyperarousal, fear memory formation and extinction, as well as restricted range of affect.26 These findings on remote trauma correspond with this study’s findings from the SOF-2wS group.
One of the limitations of the present study is that it only included male participants. Excluding female participants was not an intentional decision but a result of the specific population studied. As women worldwide are at an increased risk for developing PTSD symptoms due to being more frequently targeted for sexual violence (e.g., rape, sexual abuse), the inclusion of female Mexican Special Forces members would have strengthened these findings. On this study the type of exercise was not register. Thus, interpretations from these findings should be presented cautiously when being generalized beyond men.
Conclusion
The BDNF levels decrease after two weeks of operational military stress exposure, also could relate to the PTSD such those group 2 participants growth PTSD symptoms, the physical training increase BDNF levels (SOF-TC). These preliminary findings suggest that evaluating BDNF levels could be an accurate method to use to in determining mental health diagnosis in male military members. Additional research is needed.











 nueva página del texto (beta)
nueva página del texto (beta)