INTRODUCTION
Currently, bone diseases are estimated to be one of the main causes of disability in humans. Osteoporosis, for example, causes approximately 9 million bone fractures worldwide with a high risk of morbidity and mortality of 12-20 % of sufferers [1]. A bone replacement must imitate the tissue by replicating its structural and functional requirements, in such a way that it generates an adequate response from the biological system to which it has been subjected [2]. To achieve this, the use of tissue engineering (TE) has been applied, which is a multidisciplinary science that uses the principles of engineering and life sciences to improve or develop biomaterials that generate a suitable microenvironment through the combination of cell [3]. The objective of TE is to produce biomaterials that are biocompatible, biodegradable, bioactive, capable of promoting cell proliferation, non-toxic, and osteoconductive, in order to provide similar characteristics to the cellular configuration of human tissue [4] [5].
Hence, several researches have developed biomaterials capable of satisfying this type of needs through the use of composite materials based on ceramics and biopolymers, since they have been shown to have a great similarity with bone tissue, since it consists of an inorganic phase (minerals such as calcium phosphate) and an organic phase (collagen and proteins) [6].
In this paper, given the importance of the development of this type of biomaterials, an extensive literature review has been carried out about the most utilized biopolymers in composites with inorganic hydroxyapatite phase, which have potential biomedical applications, describing the generalities of each of them, as well as recent research on this type of biomaterials.
MATERIALS AND METHODS
A bibliographic review was carried out to collect information about hydroxyapatite, biopolymers, as well as biopolymer/hydroxyapatite composites using the keywords hydroxyapatite, biopolymers, polymer matrix composites, tissue engineering, composite procurement, etc. Original papers and books were taken into account, both in English and Spanish, published in scientific journals indexed in Scopus, Science Direct, Google Scholar, and Web of Science. No limits on the year of publication were used, but the articles published in the last ten years were chosen for the description of how biopolymer/ hydroxyapatite composites are obtained, selecting a total of 132 references including both articles and books.
Hydroxyapatite
The skeleton of the average adult is composed of 40% bone structure with size and shape defined for specific functions, the most important of which are to facilitate movement, protect vital organs, store minerals, deposit bone marrow, and provide the support structure of the body [7] [8]. Human bone is a complex dynamic tissue of dense nature, its extracellular matrix is composed mainly of collagen fiber (organic phase 30%) and the mineral part of which the major component is calcium phosphates (inorganic phase 70%). The inorganic content of the bone matrix is hydroxyapatite (Ha), which in combination with other biomaterials has been demonstrated to provide biocompatibility, biodegradability, and osteoconductivity [4] [9].
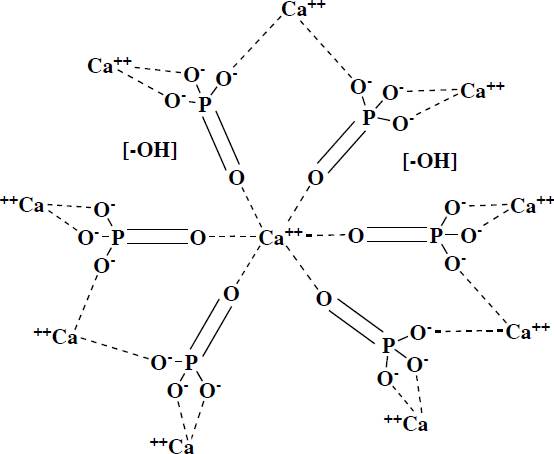
Ha is closely related to basic calcium phosphate, which is the predominant inorganic material in the bones of all vertebrates, as well as in dental enamel. Has the chemical formula Ca10(PO4)6(OH)2, this phosphate crystallizes in a hexagonal form, see Figure 1, with a P63/m symmetry group, with lattice parameters a = b= 9.432 Å and c = 6. 88 Å, it is commonly obtained in aqueous conditions and often tends to have particle sizes around 5 to 1 nm, so they have a large surface area, which intensifies its chemical activity. The ionic character of the Ha makes it a hard ceramic, refractory, with a melting point above 1600 C [10] [11] [12].
Ha's biomedical applications have been of great relevance due to their high degree of biocompatibility and bioactivity. Has been employed in the manufacture of different scaffold systems, including porous structures and hydrogels, with potential applications for the regeneration of bone tissue [13], and several methods have been developed to obtain this material, the main ones being precipitation, sol-gel, mechanochemistry, and solid-state reaction.
Ha can be obtained from natural sources, the most well-known are eggshells, corals, and animal bones, through thermal treatments at high temperatures [14]. Figure 2 provides a general description of the methodologies used to obtain Ha.
Biopolymers
The prefix "bio" indicates that these polymers are obtained from living matter or natural sources such as animals, plants, and microorganisms. They possess diverse characteristics such as biodegradability, low toxicity, biocompatibility, etc., which make them good candidates for a wide range of applications ranging from food and textiles up to electronics and medicine [15].
However, the term "biopolymer" is not strictly defined in the literature. There is some ambiguity in the term’s biopolymers, biodegradable polymers, bio-based polymers and bioplastics [16]. Biopolymers are those polymers that are derived from natural resources and exhibit biodegradability, but do not include synthetic polymers that are biodegradable, they are also often referred to as natural polymers [17]. Biodegradable polymers include polymers of both natural and synthetic origin and are also known as bioresorbable polymers [18]. Bio-based polymers are those that are obtained partially or totally from renewable resources or waste and can be biodegradable or non-biodegradable [19]. And bioplastics can become biodegradable even if they are not necessarily derived from natural sources [20].
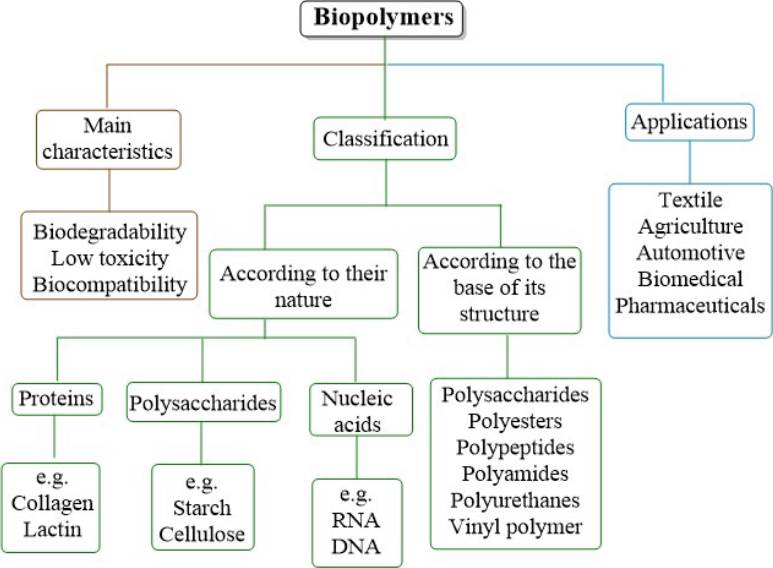
Biopolymers have been designed by nature itself with a single purpose, the sustenance of life. They can be grouped in different ways [21], as illustrated in Figure 3. Based on their nature, they are classified into three main categories: polysaccharides, proteins, and nucleic acids, where polysaccharides are macromolecular chains of low molecular weight that are linked by covalent sugar bonds, the best-known being glucose compounds or their derivatives such as starch and cellulose, which make the structure of plants. The proteins are the most important macromolecules present in animals, these are formed by the polymerization of amino acids, each type of protein has its own sequence of amino acids and structure which make its purpose determined, the most well-known proteins are hemoglobin, collagen, albumin, lactin, among others. Nucleic acids are linear polymers formed mainly of sugar molecules, phosphates, and bases, the most mentioned are RNA and DNA, which are found in living organisms for the purpose of storing and reading genetic information [22] [23] [24]. Depending based on their polymeric structure are polysaccharides, polyesters, polypeptides, polyamides, polyurethanes, and vinyl polymers [25].
Due to the characteristics of biopolymers, they have become a tendency for their use in diverse applications, among which the pharmaceutical and biomedical areas stand out [22]. For this reason, the design of new materials based on biopolymers or biomaterials that include a composite of a polymer and a material in order to be compatible with human tissue, is one of the most promising areas in reconstructive medicine and orthopedics. In the case of implants made from biopolymers, they are used as a temporary osteoconductive scaffold, to which new bone tissue, compatible with the biopolymer, will eventually be anchored and grow. One of the greatest advantages of biopolymers in this area is that the implants do not require additional surgical intervention to remove them from the organism. Although one limitation they may have is their low mechanical properties since their high Young's modulus does not allow them to maintain the morphology of the polymeric implants under the load suffered by the musculoskeletal system [26] [27] [28]. Biopolymers, therefore, tend to have a wide field of utility, since they can be used for prostheses and implants, bone cements, and components of artificial organs [29].
Composites
Composites are materials consisting of two phases, illustrated in Figure 4, where the first is called the discontinuous or dispersed phase (reinforcement or filler) and the second is the continuous or "matrix" phase. The reinforcements or fillers can be fibers, particles, or sheets, whereas the matrix is the predominant phase [30].
Composite materials have originated since old times, human beings have used them for different purposes, such as hunting weapons [31], in the same way nature provides these materials, for example, wood is composed of cellulose fibers in a lignin matrix, or the bones of mammals are constituted by collagen fibers in a calcium phosphate matrix [32].
These materials are developed to enhance their properties in relation to their pure components. The most desirable characteristics of these composites are their physicochemical properties, such as resistance to chemical attack, high mechanical properties, as well as favoring their use in everyday life. In the development of these composites, the aim has been to combine the properties of two or more components in a single compound, as well as to generate energy savings both in their production and application [33]. Based on this range of qualities, composites are considered as a feasible replacement for conventional materials in several industries such as aerospace, automotive, medical, among others [34].
Biopolymers and calcium phosphate salts composites have been used for bone replacement, because biopolymers are a good substitute for synthetic polymers, in addition to the fact that their composition can imitate the extracellular matrix due to the fact that they are bioabsorbable, biocompatible, and biodegradable. This type of materials with biomedical applications must have a great similarity with the structure and composition of the components present in the molecular structure of living beings; in this sense, proteins and polysaccharides are widely used in TE and regenerative medicine [35]. Therefore, the following sections will describe biopolymer/hydroxyapatite composites with potential biomedical applications, focusing on the most widely used for this purpose.
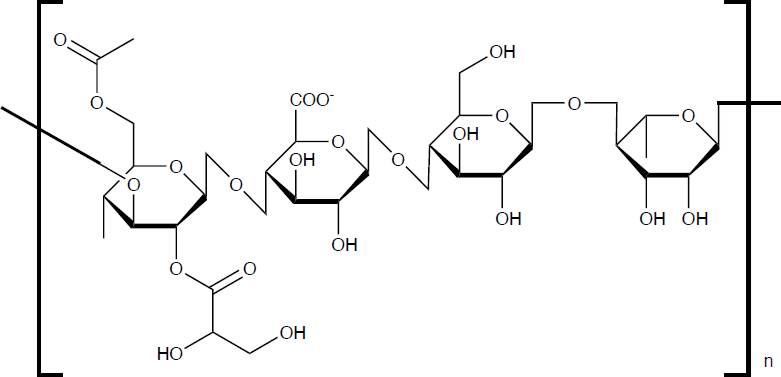
Alginate/Hydroxyapatite (Alg/Ha)
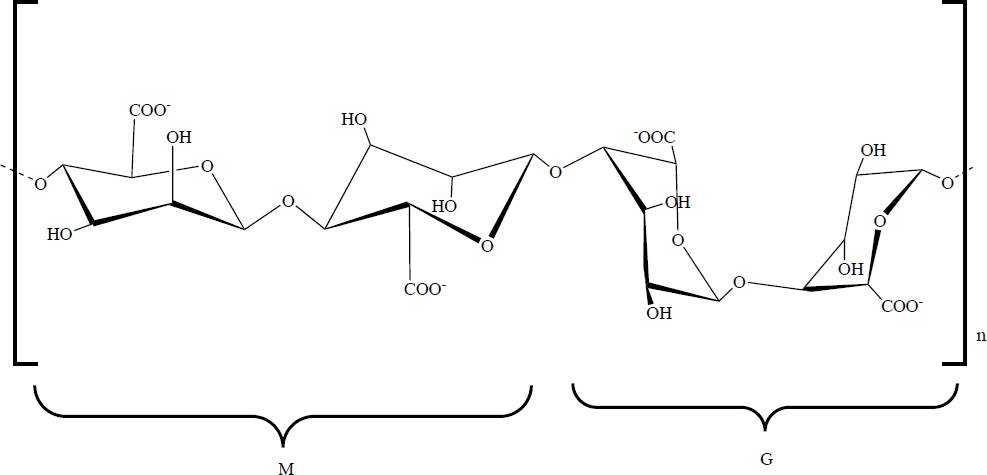
Alginic acid, also called alginate (Alg), occurs in nature mainly in brown algae (Phaeophyceae). Alg is an non-branched linear polysaccharide composed of the monomers β(1-4) D-mannuronic acid and α(1-4) L-guluronic acid, referred to as M- and G-block, respectively [36], its chemical structure is shown in Figure 5. Alg has diverse properties such as biodegradability, biocompatibility, bioadhesiveness, low toxicity, hydrophilicity, non-immunogenic effects, low cost, in addition to the fact that with the presence, even in low concentrations, of different cations (K+, Na+, Ca2+, Mg2+, Ba2+, and Sr2+) a stable hydrogel can be formed, due to the carboxylate groups of Alg [37]. In recent years, Alg has been reported to be utilized in medicine as a surgical and wound dressing [38], as a drug releasing agent and as a scaffold for bone, cartilage and/or skin [39]. These characteristics have led to it being widely studied as it is considered suitable for use in TE due to its biocompatibility with living cells and its property for the formation of hydrogels [40].
With respect to Alg/Ha composites, these have been prepared by various processes and techniques, such as freeze-drying, electrospinning, extrusion, layer-bylayer, uniaxial hot pressing, liquid-gas systems, impregnation, coprecipitation [41], but the method most commonly used to obtaining them is crosslinking, however, it generally leads to a lack of homogeneity causing a decrease in mechanical properties [42].
Different researchers have developed this type of composites, such as that carried out in 2021 by Ocando et al., who report obtaining an Alg/Ha composite in which Ha was doped with Mg, since it plays an important role in the proliferation of osteoblasts. In this work they obtained a biomaterial, by means of the crosslinking method, highly porous, with a uniform morphology, good biocompatibility and bioactivity [43].
This type of composite provides an improvement in different properties like mechanical strength, porosity, cell adhesion, biocompatibility, excellent osteogenic differentiation and mineralization [44], good bone regeneration, and can be used in the fixation of bone implants [45]. Table 1 shows the description of Alg/Ha systems where in some cases there are three-phase systems.
Table 1 Description and application of Alg/Ha systems.
| Ref. | |||
|---|---|---|---|
| Alg/Ha-DS | Drug release | The in-situ production of Ha into the Alg polymeric matrix allowed for reduced swelling and dissolution rate. It was successfully applied to the release of the drug diclofenac sodium (DS) and a controlled release of up to 8 h was obtained. | [97] |
| Alg/Ha-CHX | Drug release in dentistry | The composite demonstrated good stability due to electrostatic interactions between chlorhexidine (CHX), Ha and Alg. In addition, a controlled release of CHX was achieved, obtaining the highest concentration at 72 h. | [98] |
| Alg/Ha | Implant | This composite developed was shown to have biological control within the subject under study, and the bone formed around the biomaterial had a lamellar structure, demonstrating that the biomaterial induces rapid bone production. | [99] |
| Alg/Ha | Biomedical application | The Alg/Ha composite tended to have better bioresorption at a higher weight percentage of Alg. An improvement in mechanical properties was also found compared to pure Ha and Alg. | [100] |
| Alg/Ha/MWCNT | Tissue engineering | The developed a composite with high porosity and showed improved compressive strength (72.02 ± 1.7 kPa). In addition, improved biocompatibility, differentiation and cell attachment were observed in the MG-63 cell line. | [101] |
| Alg/Ha | Tissue engineering | The obtained a composite that proved to have good biocompatibility and biodegradability. A histological study determined that the scaffolds developed can support both endochondral and intramembranous bone formation, and the defect was completely regenerated after 6 months. | [102] |
| Alg/Ha | Coatings | This composite showed improved mechanical properties and in microbiological studies they found that the composite significantly reduced the growth of the gram-positive bacterial L. monocytogenes compared to the pure Alg. | [103] |
Collagen/Hydroxyapatite (Col/Ha)
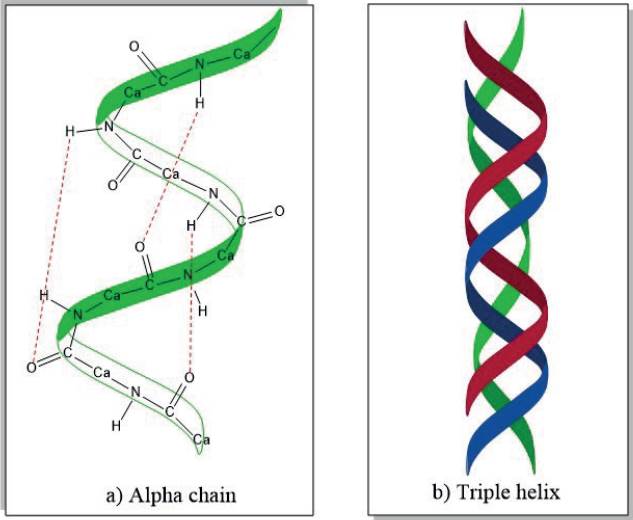
Collagen (Col), specifically type I, is the most abundant protein in the human body and can be found in different mammalian tissues such as the cornea, cartilage, skin, bones, tendons, ligaments, and others. It provides mechanical strength and stimulates cell adhesion and proliferation [36]. Is arranged as a triple helical structure which is mostly stabilized by hydrogen bridges [46] as shown in Figure 6. Has several properties that make it unique such as biodegradability, non-immunogenicity, bioabsorbability, and biocompatibility [47] [48].
Col has been used in TE and regenerative medicine for many years due to its biocompatibility and low immunogenicity, however, by itself it is not naturally osteoconductive and tends to have low compressive strength [49]. Col/Ha composites have been successfully used in tissue procurement, since this combination provides good mechanical properties and osteoconductivity, in addition to favoring cell adhesion, cell migration, vascularization and osteoblastic differentiation [50]. In the same way, several researchers have opted for Col as the polymeric matrix par excellence in this type of composites since it is naturally present in the composition of human bones [51].
Col/Ha composites have been commonly obtained by different methods [52] [53] [54]:
Mixing in collagen solution with Ha powder and subsequent lyophilization.
Co-Precipitation of Ca and P precursors in collagen solution.
Crosslinking for hydrogel formation.
Mechanical mixing.
Electrochemical deposition.
Studies realized in 2018 by Kaczmarek et al., demonstrated that this type of composites shows an increment in osteoconductivity, mechanical properties, and biocompatibility [55], in the same sense these authors also found with this composite a high cell proliferation and a high mineralization potential [56] [57].
Similarly, these biomaterials have a great potential to be applied in regenerative medicine through implants and/or bone tissue regenerator [58]. In addition, it can be used as a controlled drug release system thanks to its absorbent properties [59]. For a clearer comprehension, Table 2 presents a brief overview of Col/Ha systems.
Table 2 Description of Col/Ha systems.
| Ref. | |||
|---|---|---|---|
| Col/Ha | Tissue engineering | The composite has a bone composition similar to human bone and was shown to have superior mechanical properties compared to pure Col, thus providing reinforcement at the interphase. | [104] |
| Col/Ha | Tissue engineering | The developed composite showed to be doubled in strength with respect to its mechanical properties, thanks to the strengthening of the chemical bonds that occur between the Col-Ha, in addition, the scaffold maintained fibrillar structures and appropriate porosity, which permits a good cell proliferation. | [105] |
| Col/Ha/Graphene | Coatings | A composite with a composition very similar to that of human bone was developed and successfully electrodeposited on the surface of a Ti16Nb alloy. This coating provided a hydrophilic surface suitable for fibroblast cell development and adhesion. | [106] |
| Col/Ha | Bone defects | The composite produced adequately retains some trace elements that help its biocompatibility, thanks to the Ha synthesized from bovine bones, so it has a good bioactivity and can be used as a bone repair material. | [107] |
| Col/Ha/CNT | Bone replacement | This composite increased the flexural strength and fracture toughness up to 3%, and also exhibited positive osteoblast cell growth, thus improving its biological properties. | [108] |
| Col/Ha | Implant and bone regeneration | The composite exhibited a cell viability of 108.9 %, inferring that Ha and Col are biocompatible and non-toxic, since a viability above 100 % indicates cell proliferation. | [109] |
| Col/Ha | Tissue engineering | In the development of this composite obtained a high porosity of between 85-90 %, they also found an improvement in the compressive modulus obtaining a value over 1MPa, they also demonstrated that the architecture, permeability and composition of the scaffold favor infiltration and differentiation in vitro after subcutaneous implantation, proving that this type of scaffolds are osteoinductive. | [110] |
| Col/Ha | Tissue engineering | The composite shows good swelling properties, having that crosslinking and concentration of Col and Ha is important to modify the microstructure, mechanical properties and degradation rate of the composite. | [111] |
Gellan Gum/Hydroxyapatite (GG/Ha)
Natural gums can be obtained from different sources such as vegetable fibers (gum arabic, gum ghatti, gum tragacanth, etc.), seeds (guar gum, konjac gum, etc.), algae (agar-agar gum, alginates, etc.), or microorganisms (gellan gum, xanthan gum, rhamsan gum, etc.) [60].
Microbial gums are of high molecular weight, approximately 1,000,000 Da, and are composed of carbohydrates, proteins, lipids, and humic substances. Are produced by certain microorganisms from the aerobic fermentation of sugar [61], Gellan gum (GG) is an exopolysaccharide of anionic nature consisting of a repeating unit of a tetrasaccharide (D-glucose, 1-3, glucuronic acid, D-glucose and 1,4, L-rhamnose), see Figure 7.
Similar to Alg, GG forms gels in presence of metal ions and can be easily processed [36], some of the main characteristics of GG are its non-toxicity, biocompatibility, and biodegradability.
This polysaccharide is produced by the excretion of the bacteria Sphingonomas elodea and Sphingomonas paucimobilis. In addition, is highly thermally stable and the gels obtained are mainly translucent [62] and have high porosity, antibacterial nature, proliferation, and bone formation [63]. They are commonly prepared by lyophilization, rehydration, and co-precipitation for the formation of cellular adhesive sponge-type hydrogels [64]. Diverse studies have shown that the gelation temperature, strength, texture, clarity, and speed of gel formation depend directly on the pH value during the obtaining process [60].
Due to its characteristics, GG has been used in diverse applications in the food, cosmetic and pharmaceutical industries; in the last of these, its application in ophthalmology, drug release, cell immobilization, biosensor synthesis, among others, stands out [64]. In recent decades, GG has been studied for numerous applications in cellular tissue, including brain TE, dentistry, cartilage, and intervertebral disc [65]. Shin et al. employed the photoreticulation reaction in order to obtain rigid microgels of GG. The product obtained was immersed in a modified gelatin solution, which was photoreticulated, and the final material showed increased mechanical strength [66].
On the other hand, several studies report that it is possible to increase the mechanical strength of GG by adding calcium phosphate salts, such as Ha. These inorganic particles can be easily incorporated into hydrogels by mechanical mixing [67]. Previous studies have shown that mineralization of GG hydrogels could enhance mechanical properties, as well as promote cell adhesion and growth, and osteogenic differentiation [68].
Recent research by Pereira et al. at 2018 successfully reported the obtaining of a GG/Ha composite by incorporating different concentrations of Ha (5, 10, 15, and 20 %) using the crosslinking method, obtaining a material with improved mechanical properties, high stability in degradation and promoting high cell proliferation, null toxicity, and excellent biocompatibility [69]. The Table 3 shows the description of GG/Ha composites.
Table 3 Description of GG/Ha systems.
| Ref. | |||
|---|---|---|---|
| GG/Ha | Bone repairment | The addition of Ha in the polymeric matrix of GG is reported to favor mechanical strength. | [112] |
| GG/Ha | Bone regeneration | This composite was made in 3D which allowed a maturation of the bone cells because it assimilates the structure of human bone. | [113] |
| GG/Ha | Bone reconstruction | The obtained composites showed high mechanical strength, good porosity with the capacity to absorb nutrients necessary for proper cell growth, as well as slow degradation, which provides the necessary time to induce osteoconduction and the generation of new bone. | [114] |
| GG/Ha | Bone replacements | The addition of Ha in the GG polymer matrix provides good cell adhesion on the surface of the material. in addition, it was observed that increasing the percentage of Ha significantly increased the proliferation of MC3T3E1 cells. | [115] |
| GG/Ha | Bone grafting | The developed material improved its mechanical properties with respect to pure GG, in addition to showing a clear organization of the cytoskeleton and cell motility from day 7. Hence, this composite favors cell adhesion and propagation. | [116] |
| GG/Ha-Ce | Bone grafting | A composite of GG/Ha developed with the addition of doped cerium (Ce) to the hydroxyapatite. This material demonstrated high porosity, increased mechanical properties, and demonstrated that it is non-toxic and biocompatible. | [117] |
Chitosan/Hydroxyapatite (Ch/Ha)
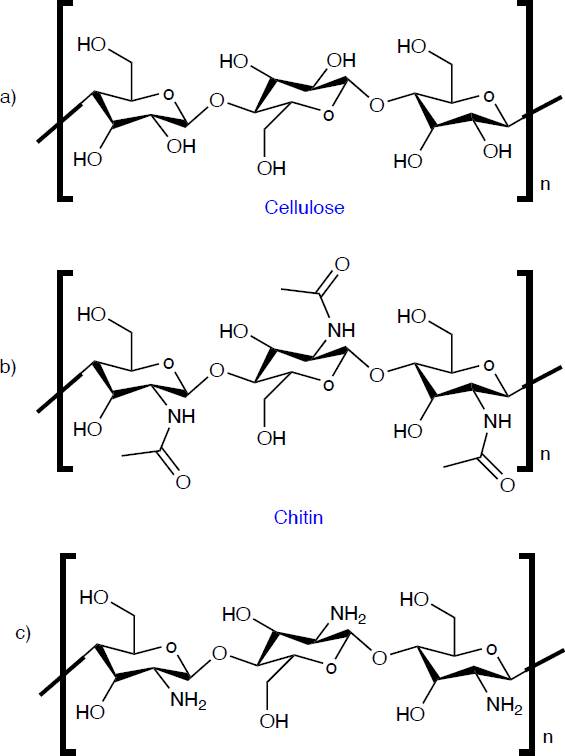
Chitin is the second most abundant organic compound on planet earth, it is a biopolymer found in various living organisms, such as the animal, plant, and fungi kingdoms. It is found in the exoskeleton of crustaceans, the cuticle of insects, and cell walls of fungi, for example, in shrimp, lobsters, crabs, squid, mollusks, scorpions, ants, beetles, cockroaches, bees, spiders, Agaricus bisporus, Aspergillus niger, Auricularia auriculajudae, and others. Chitin is an effective material for suturing, wound healing and drug release thanks to its biocompatibility, non-toxicity, immunogenicity, biodegradability and antimicrobial activity [70]. Although the most important aspect of chitin is its main derivative, chitosan (Ch), which is obtained by deacetylation of chitin. Chemically, Ch has a similar structure to cellulose and chitin, as shown in Figure 8, its differentiator is the functional group at the C-2 position. While cellulose has a hydroxyl group (-OH) and chitin has an N-acetylamine group (-NHCOOH3), Ch has an amino group (-NH2). Ch is a linear polysaccharide consisting of an acetylated unit (N-acetyl-2-amino-2deoxy-d-glucopyranose) and a deacetylated unit (2-amino-2-deoxy-glucopyranose), where the repetitive units are linked by β(1-4) glycosidic bonds [71]. The degree of deacetylation and molecular weight are of major influence on the physicochemical properties of Ch, where the degree of deacetylation represents the ratio of glucosamine to N-acetyl-glusoamine [72].
Non-toxicity, biodegradability, biocompatibility, antimicrobial activity, anticancer activity, antiinflammatory, hydrophilicity, crystallinity, immunogenicity, cell adhesion, cell proliferation and differentiation, and osteoconductivity [73] [74] [75] are some of the properties of Ch which make it unique. For this reason, it has been used in countless applications in the food industry, agriculture, pharmacy, medicine, dentistry, ophthalmology, cosmetics, the textile industry, veterinary medicine, wastewater treatment, and has also been used in the formulation of fire-retardant foams, among others [71] [76] [77]. Ch has been shown to be suitable for use in IT as a bone tissue graft, in the manufacture of porous scaffolds, films for superficial wounds, drug release, and in formulations for the production of hydrogels [78].
However, Ch presents as a disadvantage its poor mechanical properties [79], so to improve this, several investigations report the development of composites based on Ch/Ha because both biomaterials present excellent biocompatibility with human cellular tissue [80], it is reported that this composite presents improvements in its mechanical properties, due to the binding of the amino and hydroxyl groups of Ch to the calcium ions present in Ha [81] [82]. The Ch/Ha composite can be produced mainly by freeze-drying; this technique is one of the most widely used to obtain scaffolds, and other techniques can also be used, such as NSN textile methodology for the production of fibrous structures and the use of enzymes. This composite can be used as a bioactive interface between the metallic implant and the human bone [75]. In 2017 Okada et al., used the coagulation method to obtain a Ch/Ha composite fiber, where they reported that this material improves the mechanical properties [83]. Also, Balagangadharan et al., reported in 2018 the development of a Ch/Ha composite to which they added ZrO2 nanoparticles obtaining scaffolds, which presented an osteoconductive improvement and osteoblast differentiation, resulting in a beneficial composite for bone tissue regeneration [84]. Descriptions of some of the Ch/Ha systems are presented in Table 4.
Table 4 Description of Ch/Ha systems.
| Ref. | |||
|---|---|---|---|
| Ch/Ha/Magnetite | Superficial wounds | The Ch/Ha composite increases the bending modulus up to 2.6 GPa, with the addition of magnetite, however, it showed a lower bending strength. In contrast, magnetic nanoparticles had no negative effect on the cytocompatibility of the material. | [118] |
| Ch/Ha | Coatings | The composites developed showed good swelling and crosslinking properties, and the L929 and Saos-2 cell lines showed cell proliferation. | [119] |
| Ch/Ha-DOX | Drug release | This study incorporated an anticancer drug (doxorubicin, DOX) into Ha nanoparticles modifying this with Ch. The modified Ha did not show cytotoxicity in the MG-63 cell line. | [120] |
| Cu-Ch/Ha-Sr | Tissue engineering | The composite showed an improvement in the mechanical properties of Ch, as well as excellent cell infiltration, cell migration and neovascularization due to the high porosity, making them cytocompatible. | [121] |
| MWCNT-Ch/Ha | Bone replacements | The nanocomposite exhibited excellent mechanical properties, good biocompatibility and bioactivity, hence fulfilling the required properties of the extracellular matrix of bone. | [122] |
| Ch/Ha | Bone replacements and coatings | The composite had a tensile strength of 30-70 MPa, showed to be cytocompatible in the L929 cell line after 24 h of incubation. | [123] |
| Ch/HaNPsAg | Drug release and implant | This type of scaffolds exhibited an improvement in mechanical resistance, as well as improved cell penetration, adhesion and propagation. The presence of silver nanoparticles (NPsAg) provides antibacterial activity, thus reducing the risk of infections. | [124] |
| Ch/Ha | Tissue engineering | The composite showed a high similarity compared to natural bone. Also observed that increasing the concentration of Ha resulted in a higher adhesion of osteoblasts. In addition, this material showed a high degree of proliferation in the MC3T3-E1 cell line. Compared to the pure Ch scaffold, the compressive strength increased by 33.07 %. | [125] |
Polylactic Acid-Hydroxyapatite (PLLA/Ha)
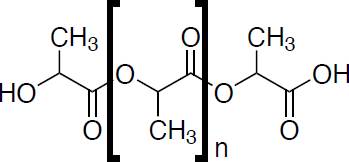
Polylactic acid (PLLA), its chemical structure can be seen in Figure 9, is a linear aliphatic thermoplastic polyester derived 100% from renewable resources with wide availability of raw materials (sugar, corn, potatoes, sugarcane, sugar beet, among many others) [85]. This material is simple to process, using any technique, such as extrusion, injection and compression molding, thermoforming and foaming, as well as blow molding, electrospinning, spinning, and stretching of molten polymer, among others. Extrusion is commonly used for PLA processing because it can be adapted for various applications [86], The industry most known is packaging; in fact, it is approved by the United States Food and Drug Administration (FDA) [87], for its excellent properties of transparency, low toxicity, versatility, osteoconductivity, biodegradability, biocompatibility, bioresorbability [88], properties influenced by its molecular weight and stereochemistry [89].
PLLA exists in two stereoisomeric forms D-PLLA (DPLLA) and L-PLLA (PLLA), and a mixture of D- and L-lactic acid also exists as D,L-PLLA (PDLLA). Polymers derived from the optically active D- and L-monomers are semi-crystalline and those derived from the optically inactive D,L-PLLA are amorphous. Generally, L-PLLA is preferred for applications where high mechanical strength and toughness are required, for example, orthopedic devices and sutures. On the other hand, due to the amorphous nature of D,L-PLLA is considered for drug release applications, since for this purpose it is very important to have a homogeneous dispersion of the active species within a matrix. It has been reported that PLLA degradation is significantly affected with increasing crystallinity, so the mixture of PLLA and PDLLA is effective in adjusting the crystallization and morphology of the polymer, and thus its mechanical properties [90]. In recent years, it has been successfully used as a surgical implant material, drug release, and in the fabrication of 3D printed scaffolds [91].
A disadvantage that PLLA may present is the absence of the property that facilitates cell binding on its surface and its proliferation, due to a scarcity of cell binding capacity [92]. This deficiency in cohesion could be improved by employing the addition of Ha as a strategy since calcium phosphates promote cell proliferation on the scaffold surface. It has also been shown that PLA/ Ha composites tend to enhance their overall properties such as biocompatibility, resorbability, osteoconductivity, cell proliferation, and mechanical strength [93].
The dispersion of Ha on PLA is considered one of the critical factors that determine the properties of the composite, for which different methods and techniques are used to obtain it, highlighting melt mixing, electrospinning, processing by compression, emulsion and solvent casting, the latter being the most used [94]. On the other hand, Zhang et al., reported in 2015 the obtaining of a PLA/Ha composite by the electrospinning method, which led to an easily obtained material with great potential in terms of its biomedical applications such as rapid mineralization and high biocompatibility [95]. Also, at 2019 de Moura et al., obtained a PLA/Ha material with improved mechanical resistance and bioactivity, reported that the addition of Ha increases the protein adsorption capacity, suppresses cell death by apoptosis, and creates a favorable microenvironment for cell proliferation [96]. The Table 5 present a description of the PLLA/Ha systems and their potential application.
Table 5 Description of PLLA/Ha systems.
| Ref. | |||
|---|---|---|---|
| PLLA/Ha | Bone replacements | The composite had an increase in Young's modulus incorporating a low quantity of Ha in the polymeric matrix. It was also demonstrated that the material is not cytotoxic in the MC3T3-E1 cell line, in besides having a good osteoblastic maturation. | [126] |
| PLLA/Ha | Bone tissue engineering | The developed composite has a hydrophilic part of the Ha, which contributes to cell growth and bone repair, as well as a hydrophobic part of the PLLA, which acts as a barrier between tissues. As a result, this material has a high cytocompatibility. | [127] |
| PLLA/HaNPsAg | Coatings | The antimicrobial activity of the composite exhibited better inhibition efficacy on E. coli bacteria. In the hemocompatibility assay showed 2 % hemolytic activity, so it is considered compatible with human blood. | [128] |
| PLLA/Ha | Coatings | The composite shown that the mechanical properties and thermal behavior increased with the addition of Ha to the PLLA matrix. In addition, the material exhibited good biocompatibility. | [129] |
| PLLA/Ha | Scaffold | The composite demonstrated mechanical properties comparable to those of normal bone. Furthermore, it exhibited excellent 3D printability at 90:10 ratio of PLLA/Ha respectively. | [130] |
| PLLA/Ha | Scaffold | The composite demonstrated good adhesion and cellular proliferation in the material, with adequate osteoconductivity. | [131] |
| PLLA/Ha | Scaffold | The composite showed good adhesion and cell proliferation on the surface, adequate porosity, as well as an increase in mechanical properties with respect to pure PLLA. | [132] |
CONCLUSIONS
Biopolymers are materials used in a diversity of applications due to their excellent qualities such as biodegradability, biocompatibility, and non-toxicity, therefore several researchers have studied them for their application in tissue engineering, however, these materials have poor mechanical properties. In response to this mechanical deficiency, composites based on biopolymers/calcium phosphate salts (hydroxyapatite) have been used, since it has been shown that these types of materials show improvements in their mechanical properties and biocompatibility. For this reason, these composites are a viable option for the development of biomaterials that can be used in different applications in tissue engineering, such as drug delivery, tissue implants, scaffolds, etc.











 nueva página del texto (beta)
nueva página del texto (beta)