INTRODUCTION
Diabetes mellitus is a chronic disorder defined by impaired insulin secretion or insulin resistance of target tissues, resulting in high blood glucose levels. Diabetic Foot Ulcer (DFU) is a complication of this disorder; it is defined as a full-thickness chronic wound located at a level distal to the diabetic patient [1]. DFU is responsible for amputating a lower limb in the world every 30 s [2], and any diabetic patient has a 25% chance of developing this wound [3].
The wound healing process comprises four overlapping phases:
Hemostasis: This stage aims to prevent further bleeding immediately upon injury, but in the long-term, this process provides a matrix for cells needed in later phases [4] [5] [6].
Inflammation: This stage establishes an immune barrier against pathogens [4] [6] [7]. The wound healing process is impaired in chronic wounds, typically presenting a self-perpetuating inflammation state [7] with abundant neutrophil infiltration, which increases proteases and reactive oxygen species (ROS) levels that further damage the tissue [8], thus a frequent goal on wound management is to reduce inflammation [9].
Proliferation: This phase forms a granulation tissue [6]. Consisting primarily of macrophages, fibroblasts and blood vessels, its function is to provide a barrier to infection and a matrix for cellular migration [10]. Re-epithelialization and angiogenesis also occur in this phase [11] [12] [13].
Remodeling: The remodeling process gradually transforms the granulation tissue into scar tissue [14]. The wound's tensile strength increases as the unarranged type III collagen deposited on the granulation tissue is degraded and replaced with type I collagen, organized in parallel bundles along tension lines and becomes highly cross-linked [10] [15] [16]. Other structural components of the extracellular matrix (ECM) that were primarily produced in earlier phases of wound healing diminishes as well, such as fibronectin, proteoglycans and glycosaminoglycans [5] [15].
Wound management involves using different materials such as films, sponges, fibers, nanoparticles and hydrogels [17]. Wound dressings should protect the wound from contamination and moisture loss and provide a structure that allows oxygen permeability and mimics ECM properties [17] [18]. Hydrogels are three-dimensional structures composed primarily of water and a cross-linked polymer network. Their high water content provides physical properties that resemble the ECM, giving them excellent biocompatibility [19] [20] [21].
Chitosan is a heteropolysaccharide made up of glucosamine and n-acetyl glucosamine derived from chitin [22]. It has many properties such as biocompatibility, biodegradability, antimicrobial and hemostatic capability, and bio-adhesiveness [23] [24]. Furthermore, it enhances wound healing by averting the accumulation of exudate, promoting gas exchange and drainage of the wound, and improving re-epithelization and the functions of polymorphonuclear leukocytes (PMN), macrophages and fibroblasts [17] [23] [25].
Plant extracts contain growth factors and compounds with antioxidant, antimicrobial and anti-inflammatory potential, representing a viable alternative to lessen the financial burden of long-term use of antibiotics and to be used in a topical wound treatment [26]. Aloe vera plant enhances fibroblasts and leukocyte's activity, and its wound healing mechanism has been positively correlated with glucomannan and acemannan [27] [28] [29]. Extracts of C. officinalis have shown positive effects on wound healing, enhancing the proliferation of fibroblasts and keratinocytes, increasing angiogenesis, reducing collagenase activity, and having antibacterial, anti-inflammatory and antioxidant activity [30] [31] [32].
Glucosaminoglycans (GAGs) are mainly formed by repeated units of an amino sugar attached to a uronic acid. In the early stages of healing, they are secreted to create the fundamental substance on which collagen and elastin deposition takes place to form the extracellular matrix [33] [34]. N-acetyl glucosamine (NAGA) is a monosaccharide and a basic component of hyaluronic acid and keratin sulfate on the cell surface, promotes the proliferation of keratinocytes and fibroblasts and increases the production of hyaluronic acid in the skin; it has also been successfully used to heal wounds. In the wound healing process, in the late stages of fibroplasia, the maturation of collagen fibers reduces the cellularity of the wound and increases tensile strength [10] [35]. Collagens are the most abundant protein family in the extracellular matrix. The main types of collagen present in the skin are type I (80%) and type III (15%). During the early stages of the formation of granulation tissue, the expression of Collagen III increases, from 20% to 50%. Later, during the maturation of the wound, Collagen III content decreases to normal levels, increasing the content of Collagen I, which contributes to the recovery of the tensile strength of the tissue [6] [16] [36]. In the present research article, we investigated the effect of chitosan hydrogels loaded with plant extracts on the wound healing process of full-thickness cutaneous wounds of diabetic and non-diabetic animals by analyzing wound contraction rates and the biochemical assessment (NAGA and collagen content) of wounded tissues.
MATERIALS AND METHODS
Materials
Chitosan with a 75-85% deacetylation and low molecular weight degree was purchased from Sigma-Aldrich. Aloe vera plants were purchased from a local greenhouse in Morelia and dried Calendula officinalis flowers were purchased from a local market in Morelia. All other reagents used were of analytical grade. Deionized water was used throughout this study.
Hydrogels and plant extract preparation
Chitosan solution was obtained by dissolving chitosan in an aqueous solution of 1% v/v of acetic acid, which was further mixed with a 1% m/v solution of CaCl2 in a 3:1 proportion, obtaining the chitosan hydrogel (CS) (Figure 1a). This chitosan hydrogel was mixed in a 9:1 proportion with the plant extracts, obtaining the CS+AV and CS+CO treatments. The final concentration of chitosan in the hydrogels was 35.99 mg/ml [37].
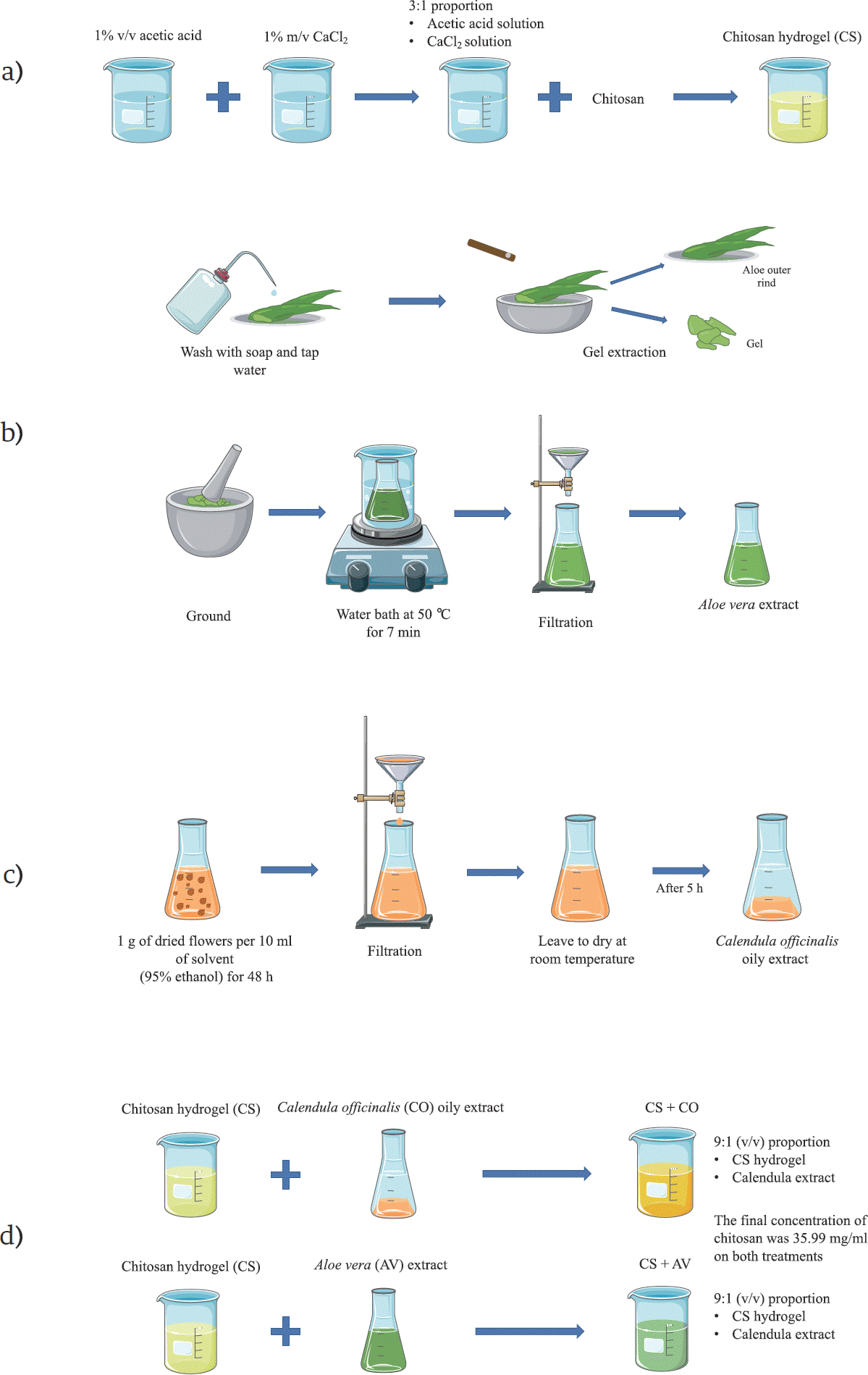
Figure 1 A scheme cartoon of the process to obtain the treatments employed in this research. (a) Process to obtain the chitosan hydrogel (CS). (b) Process to obtain the Aloe vera extract (AV). (c) Process to obtain the C. officinalis extract (CO). (d) Process to obtain the CS+AV and CS+CO hydrogels. The majority of the schematic art pieces in this figure were provided by Servier Medical Art (smart.servier.com). Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License.
Healthy leaves of Aloe vera (AV) (2 years old) were processed as previously reported [38]. Briefly, Aloe vera leaves were washed with soap and tap water, and a longitudinal incision was performed on them to extract the Aloe vera gel, which was ground and heated on a water bath at 50 °C for 7 min (Figure 1b). After the water bath, the gel was filtered using sterile gauze and immediately mixed with the CS gel (obtaining the CS+AV treatment) (Figure 1d).
A macerate of dried Calendula officinalis (CO) flowers was prepared using a 95% ethanol aqueous solution, mixing 1 g of dried flowers per 10 ml of solvent for 48 h, after which the mixing was filtered using sterile gauze. The resulting macerate was dried at room temperature for 5 h to remove the ethanol, obtaining an oil (Figure 1c) that was mixed with the CS gel (obtaining the CS+CO treatment) (Figure 1d).
In vivo wound healing study
Experimental design
In this study, a total of 24 Wistar male rats weighing between 260 and 320 g were used. The animals were kept in individual cages in a room with a constant temperature of 25 ± 1 °C with a 12 h light/dark cycle. All rats had free access to water and standard laboratory chow. All animal procedures were conducted following the Mexican Federal Regulations for Animal Experimentation and Care (NOM-062- ZOO-1999).
Diabetic model protocol
As reported in previous studies [3] [39] [40] [41], diabetes was induced after fasting for 12 h, by intraperitoneal (i.p.) injection of 45 mg/kg body weight (BW) streptozotocin dissolved in 0.1 M citrate buffer (pH 4.5). To confirm that the diabetic model was successfully established, blood was drawn by a small incision on the rat tail using a blood lancet and it was absorbed using blood glucose test strips to determine the blood glucose levels using a glucometer (AccuCheck). When the glucose level was higher than 200 mg/ml, the diabetic model was established.
Incisional wound model
Animals were anesthetized with an i.p. injection of 50 mg/kg BW pentobarbital, after which the hairs on the back of the animals were shaved using an electric clipper. A fold was created on the skin dorsum, which was then cut using a 6-mm sterilized biopsy punch, creating a full-thickness round wound on each side of the dorsum.
Animals were divided into six experimental groups (n=4), three of them with streptozotocin-induced diabetic animals:
I: diabetic control group (no treatment)
II: diabetic group topically treated with CS+AV
III: diabetic group topically treated with CS+CO
IV: non-diabetic control group (no treatment)
V: non-diabetic group topically treated with CS+AV
VI: non-diabetic group topically treated with CS+CO
Wounds were treated topically with 100 μl of the correspondent hydrogel. Each animal was monitored for 13 days from the day of injury induction (Day 0), after which the animals were euthanized by injection of a lethal dose of pentobarbital (100 mg/kg BW). Fullthickness biopsies were retrieved from the wound site for further biochemical analysis.
Wound healing analysis
Wound kinetics and data analysis
Wound kinetics were recorded using a 13 MP digital camera and were quantified using AutoCAD to measure wound area daily. The wound area was expressed as a percentage of the initial wound area (100%).
Biochemical assessment of wounds
Samples were dried at 48 °C for 24 h, after which they were hydrolyzed in 5 ml of concentrated HCl/acetic acid solution (4:1 vol/vol) at 70 - 80 °C for 3 h. After being cooled with tap water, hydrolyzates were diluted in distilled water with a final concentration of 50% v/v for use in estimating hydroxyproline (Hyp) and n-acetyl glucosamine (NAGA). Collagen content in the skin was calculated from the Hyp content by multiplying it by a factor of 7.46 [42]. The assessment was carried out using a reaction with Erhlich's reagent; briefly, a pyrrole ring was formed on both compounds (by oxidative dehydrogenation in Hyp [43] and NAGA by alkaline treatment [44] that reacts with Ehrlich's reagent to form a quinoid chromophore that can be quantified by UV-Vis spectroscopy.
Hydroxyproline and Collagen estimation. The hydrolyzates were subjected to oxidation by the addition of 1 ml of a Chloramine-T solution (1 part of Chloramine-T solution at 7% w/v and 4 parts of buffer (pH 6) of 0.42 M sodium acetate, 0.127 M sodium citrate and 0.026 M citric acid) and 1 ml of isopropanol. After 4 minutes, 2 ml of Ehrlich reagent (prepared as previously described by Cheng [45] were added. The mixtures were heated in a water bath at 60 °C for 25 min. After cooling with tap water, absorbances were read at 557 nm. A standard solution of Hydroxyproline (Hyp) was obtained by diluting 25 mg of Hyp (purchased from Sigma Aldrich) in 250 ml of water (100 μg/ml) and was used to prepare a calibration curve. Aliquots of 5, 25, 50, 75 and 100 μl were taken and diluted in distilled water to a final volume of 5 ml, obtaining solutions with 0.1, 0.5, 1, 1.5 and 2 μg/ml. These solutions were treated with the same procedure as the hydrolyzates to get a Hyp calibration curve at 557 nm. Hyp content was estimated by linear interpolation. As stated before, Collagen content was calculated by multiplying the Hyp content by a factor of 7.46.
N-acetyl glucosamine (NAGA) estimation. Briefly, 0.5 ml of the hydrolyzates solutions were added 0.5 ml of potassium tetraborate 0.1 M and heated on boiling water for 3 min; afterward, 3 ml of Ehrlich reagent (prepared as described by Reissig, Strominger & Leloir [46] were added. The mixtures were heated on a water bath at 36-38 °C for 20 min. After cooling with tap water, absorbances were read at 545 nm. The values of NAGA were estimated using the molar extinction coefficient of 21,000 [46].
RESULTS AND DISCUSSION
Results
Wound kinetics. Figures 2 and 3 show the wound kinetics and wound area ratios for both diabetic and non-diabetic groups, respectively. Both diabetic and non-diabetic groups treated with CS+AV showed a reduced wound area compared to the respective control groups from day 1 after wounding, while CS+CO treatment reduced wound area from day 1 only on the non-diabetic group in comparison to the group IV. Groups V and VI showed remarkable differences in wound area ratios compared to group IV, reducing the wound area up to 40 ~ 60 % on day 1 and resulting in a smaller wound area ratio than the control group by the end of the study. On the other hand, group II showed a decrease in wound area ratio of ~29% on day 1 and group III behaved very similarly compared to diabetic control. Nevertheless, groups III and II showed a 13 ~ 15% decrease in wound area compared to group I on the last day of the study.
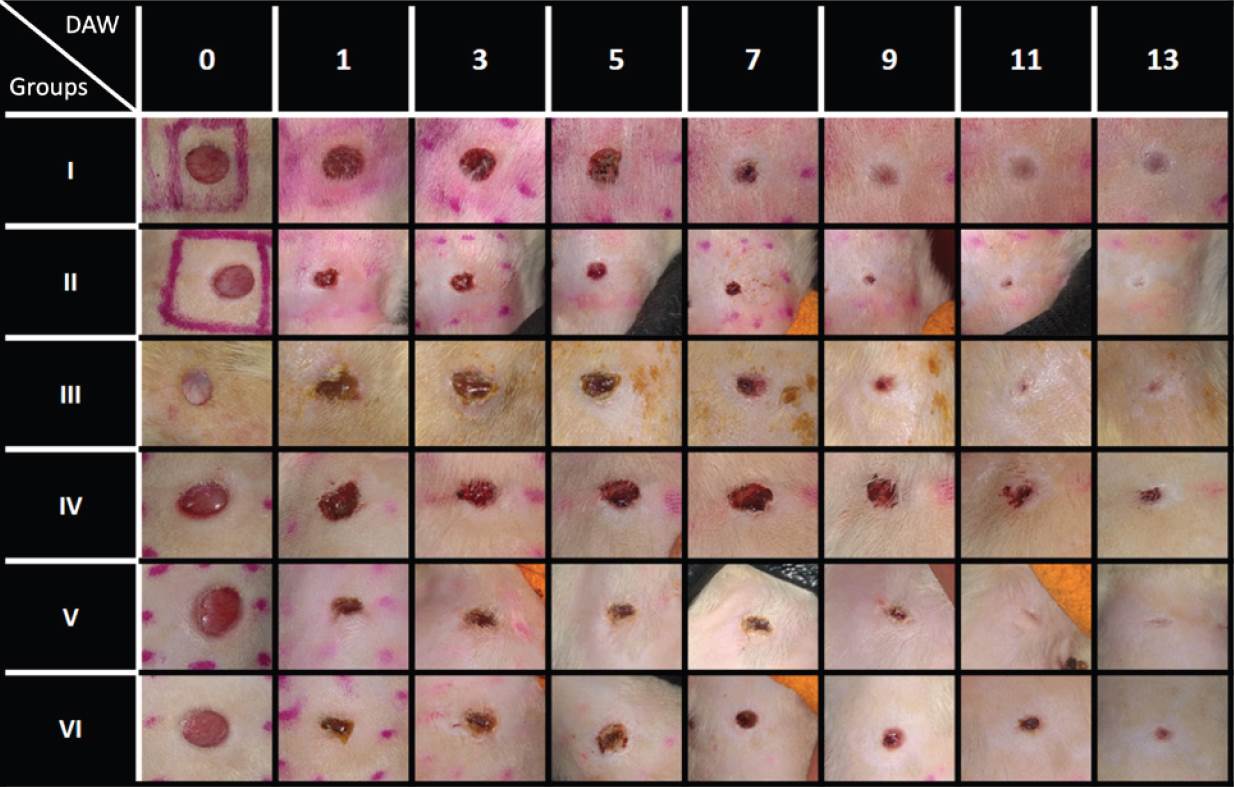
Figure 2 Wound kinetics of full-thickness wounds on various days during the healing process. DAW = Day After Wounded.
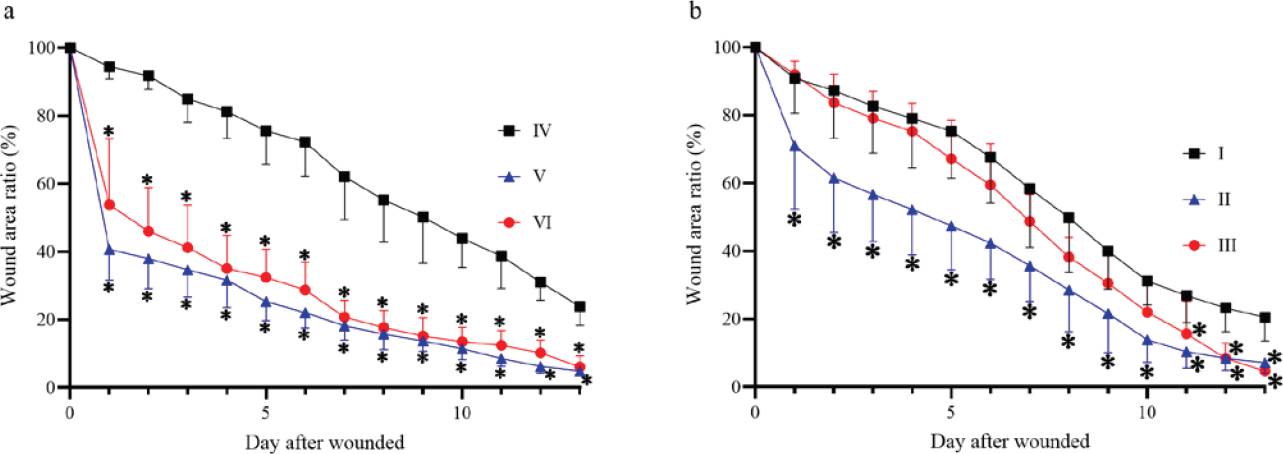
Figure 3 Wound area ratio during the wound healing process. (a) Wound area ratio on non-diabetic groups. (b) Wound area ratio on diabetic groups. *P ≤ 0.05 compared to the corresponding control group.
Hydroxyproline and Collagen estimation. Figure 4 (a and b) shows the results of the assessment of the collagen content. The groups treated, both diabetic and non-diabetic, show a higher collagen content compared to their respective control groups. Interestingly, diabetic groups show a higher content of collagen compared to the non-diabetic groups.
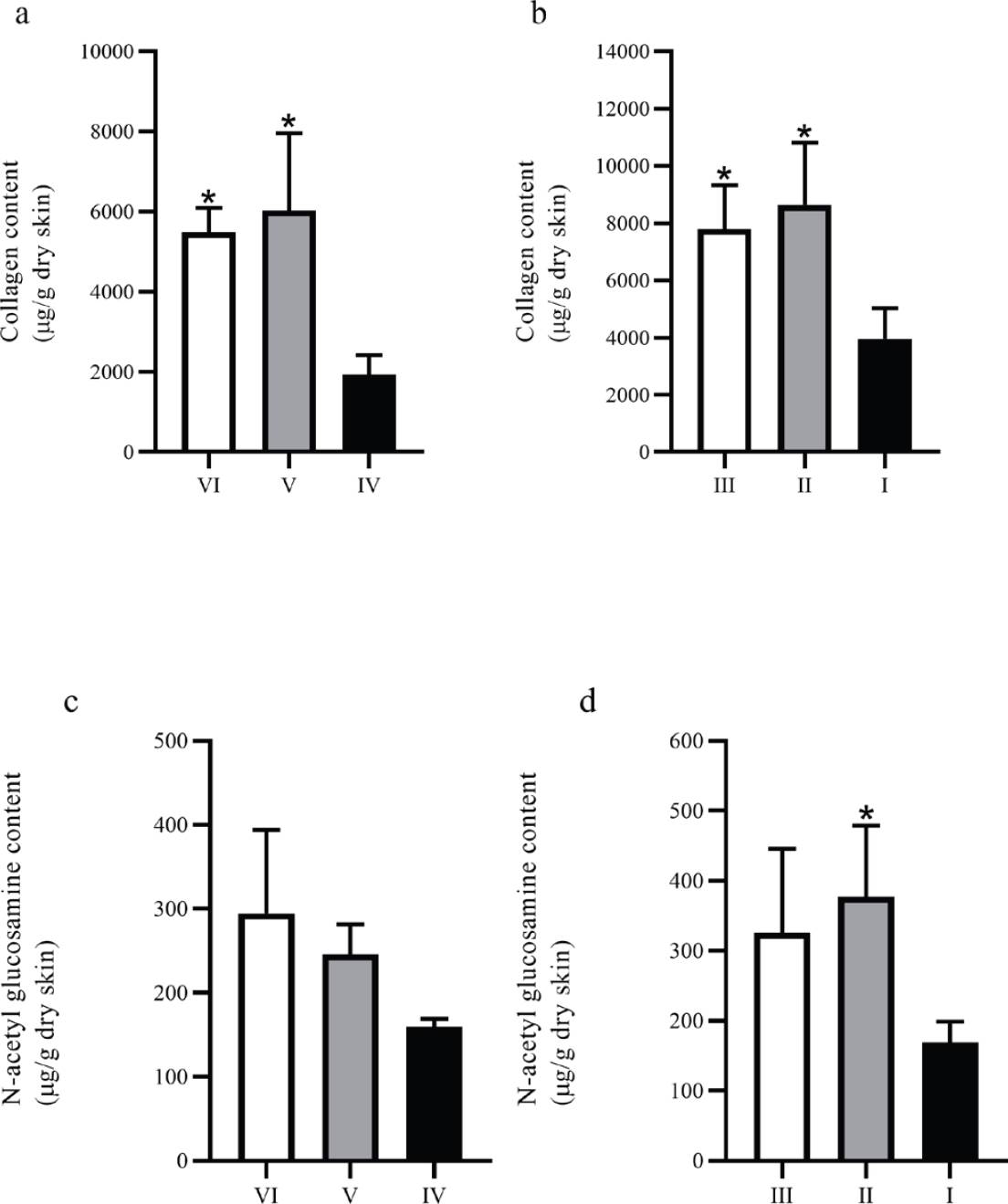
Figure 4 Collagen and NAGA content on biopsies from full-thickness wounds. (a) and (b) show the collagen content on biopsies from non-diabetic and diabetic groups, respectively; (c) and (d) show the NAGA content on biopsies from non-diabetic and diabetic groups, respectively. *P ≤ 0.05 compared to the corresponding control group.
N-acetyl glucosamine (NAGA) estimation. NAGA content increased in the groups treated with both gels compared to the control groups; however, only the diabetic group treated with CS+AV (group II) presented a statistically significant difference compared to the control group. Similarly, as in the collagen content, the diabetic groups showed a slightly higher NAGA content than the non - diabetic groups (Figure 4c and 4d).
Discussion
This study investigated the wound healing process of wounds treated with two chitosan hydrogels loaded with different plant extracts of Aloe vera and Calendula officinalis. Our results showed that treatment with both hydrogels accelerates wound contraction and enhances ECM's components' deposition, and are by previous studies involving chitosan, C. officinalis and A. vera [31] [33] [42] [47] [48]. Speed of contraction is significant in decreasing the wound's area in full-thickness injuries [49]. The quantitative analysis data of the wound area ratio suggests that the best treatment at achieving this was CS+AV since it showed better performance at reducing the wound area ratio in both diabetic and non-diabetic groups. Although the diabetic and non-diabetic control groups did not have a significant difference between them, the comparison between the groups treated with CS+CO suggests that the diabetic model has a slower wound contraction and less sensitivity to this treatment in contrast to the non-diabetic model. Myofibroblasts are differentiated fibroblasts that exhibit an actin microfilaments rich cytoskeleton and are the primarily responsible cells for wound contraction [4] [5] [7] [12]. Wound area ratio results suggest treatments might have enhanced myofibroblasts differentiation and activity in the early stages of wound healing (except on the group III, in which a significant wound contraction was only seen until day 11 after wounded). Further studies comparing the effect of chitosan and plant extracts are necessary to clarify the mechanisms and components accountable for this effect.
Accelerated wound healing can also be detected by analyzing ECM's deposition dynamics, particularly glycosaminoglycans (GAGs) and collagen [15] [50]. GAGs are mainly composed of repeating units of an amino sugar (e.g., n-acetyl glucosamine or n-acetyl galactosamine) attached to a uronic acid. During early wound healing, the ground substance is composed mainly of GAGs that regulate early inflammation, fibroblast migration and form scaffolds for collagen polymerization and maturation [16] [42] [51]. Subsequently, the content of GAGs in the tissue is reduced, and collagen production increases [33] [34]. In late fibroplasia stages, collagen fiber maturation reduces wound cellularity and increases tensile strength [10] [35] [52]. The low levels of NAGA observed in all groups in proportion to collagen content suggest that the wounds were in a middle or late stage of fibroplasia, in which collagen is mainly produced in the ECM [42] [49] [53]. It should be noted that the high collagen content observed in the treated groups may have been produced by an increase in the content of GAGs in the early stages of healing since collagen synthesis is influenced by the mucopolysaccharides of the ground substance of the ECM.
In the skin, the main types of collagen present are type I (80%) and type III (15%); the ratio of these two proteins changes during the early phases of wound healing. During the formation of granulation tissue, the expression of Collagen III increases to Collagen I expression, from 20% to 50%. Later, during wound maturation, the proportion decreases again to normal levels, increasing Collagen I's content, which contributes to the recovery of the tissue's tensile strength [6] [16] [36]. The high collagen levels observed in the treated groups suggest that the formulations improve the healing process since the increase in the collagen content in this process has been correlated with the maturation of the tissue, with the decrease in total cellularity (which is a sign of decreased inflammation) and with the development of blood vessels [42] [53]. Although collagen's overproduction could produce a keloid or hypertrophic scar [54], this pathology was not observed in either animal group of this study.
These results could be attributed to the different types of bioactive compounds present in the plant extracts that were employed. The anti-inflamatory and fibroblast stimulating activity of calendula has been attributed to its high terpenoids content, some of the identified terpenoids on calendula’s extracts are: sitosterols, ursadiol, calenduladiol esters, calendulosides, calenduladiol, faradiol, among others [30] [55] [56]. In addition to this, these extracts are also especially rich in flavonoids, like quercetin, isorhamnetin, isoquercitin, narcissin, calendoflaside, calendoflavoside and calendoflavobioside, among others. The flavonoid content confers the extracts of its antimicrobial and antioxidant properties [31] [55] [57]. Meanwhile, the Aloe vera possess 75 potential bioactive phytochemicals. Its immuno-stimulatory activity has been attributed to the polysaccharides present in the pulp [58], like glucomannan and acemannan; the former is considered as a very strong immunomodulator of plant origin, while the latter is responsible for augmenting the collagen production by enhancing fibroblast proliferation. Other type of bioactive phytochemical present in Aloe vera are anthraquinones, which have anti-inflamatory and antioxidant activity [58] [59]. Although the extracts have many bioactive compounds with different properties, it is possible that the beneficial effects of the extracts are a result of the interaction between these various compounds. Further experiments with the separate components of the treatments of this study (Chitosan, Aloe vera and Calendula officinalis) are needed to identify which component is responsible for the different effects observed. Also, the characterization of these components could help to elucidate if one chemical compound could be specifically responsible for the major benefits observed or if these are the result of a synergic effect from the various bioactive phytochemicals and the chitosan.
CONCLUSIONS
In conclusion, data collected in this study shows that chitosan hydrogels loaded with plant extracts of Aloe vera and Calendula officinalis have a beneficial effect on the wound healing process of full-thickness wounds in both diabetic and non-diabetic models. The CS+AV treatment showed a remarkable performance on the diabetic model in comparison to the CS+CO treatment. Nevertheless, the biochemical results suggest that both treatments were able to reduce inflammation and accelerate fibroplasia. Hence, this study shows that the developed hydrogels are potential materials to treat skin wounds, particularly DFU, given the observations on the treated diabetic model. Further studies are required to understand the specific effects that the treatments have on each wound-healing phase and which component of the therapy is responsible for each outcome.











 nueva página del texto (beta)
nueva página del texto (beta)



