Introduction
The teaching of life sciences is traditionally organized into two parts: theory and lab practice 1,2. At the undergraduate level, lab experiments to teach physiology and neurosciences are very expensive. Additionally, it is difficult to carry out experiments for intracellular recording with current and voltage clamp techniques. The need for space, expensive equipment, lab material and experimental animals for educational purposes is out of reach for most universities 3.
Diwakar et al. reported that in India an investment of around 20 million rupees (~267,900 USD) is required for a typical patch clamp configured for lab use, to which other costs such as the animals and facilities must be added 4. In Mexico, the presence of up to 50 students in each classroom makes this kind of lab practice impossible. One feasible alternative is the development of simulators for teaching. Indeed, simulated patients already form part of the learning environment in different disciplines of the medical field 5,6,7 and virtual microscopy practices are employed in histology 8.
Teaching the basic principles of neuroscience is of special interest and can be greatly enhanced by incorporating realistic and interactive simulations of neuronal functioning 9. Single-neuron computer simulations began with the work of Hodgkin and Huxley 10. Several simulators are now available to realistically simulate neuronal networks, including GENESIS Simulation System 11, NEURON Simulation Environment 12 and NSL Neural Simulation Language. With the increasing capacity of computational performance, the simulation of the entire brain may ultimately be possible 13.
However, only a few neurosimulators have been adapted to a teaching environment. For example, Hernández and Zurek developed a module of teaching in NEURON, allowing students to examine the properties of biophysics in the axon by using the Hodgkin-Huxley model 14. A simulator capable of reproducing the classic Hodgkin and Huxley experiments was developed by Reyes-Lazalde et al. 15. The basic study of the passive properties of the axon and dendritic tree can be carried out with an interactive simulator developed for Windows® environment 16. Brian, a program written in Phyton and found at http://brian.di.ens.fr, was developed for quickly coding models of spiking neuronal networks in everyday situations 17. iCell, an interactive cell modeling tool located at http://ssd1.bme.memphis.edu/icell/, integrates research and education for electrophysiology training. It consists of JAVA applets that represent models of a variety of cardiac cells and neurons and provide simulation data on the bioelectric activity of a single cell 18.
Traditional learning media, such as multimedia-based demonstrations, videos, reading and lectures, are passive environments and therefore limit the interactive experience of students 3. Contrarily, computer simulation enables active learning 3. The requirements are a computer room, software and an instructor. Several reports have described the pedagogical value of simulators 4. During a neuroscience course, a comparison was made between traditional teaching and active teaching aided by simulators, finding a greater understanding with the latter 3.
Ribarič and Kordaš tested the effectiveness of a software package for the study of cardiovascular physiology at the undergraduate level of a medical school. The software presented a new approach for teaching physiology, involving active learning and confronting students with multiple ways of simulating basic and clinical physiological phenomena 19. Reyes-Lazalde et al. obtained favorable results when employing software for teaching science 20. Hence, virtual labs have shown their utility for learning science 21.
During the current pandemic, teaching at a distance has added new relevance to information and communication technology (ICT). In this ICT-induced thrust, there are novel types of teacher-student interactions and new pedagogical methods. Since all lab practices are now suspended, virtual labs provide an alternative. Due to the high costs of lab practices, the government of India has sponsored an initiative to develop virtual labs, including neurophysiology labs 4.
In the present work, simulators were developed for the teaching and learning of A-type potassium ion current in neurons. They will permit students to perform virtual experiments with a current and voltage clamp. With the help of an instructor, students will be able to appreciate the importance of the A-type potassium current (IA) and how it modifies the train of action potentials (AP train), and discover other neuronal ion channels in addition to those described in the axon.
In 1961, Hagiwara et al. 22 recorded IA for the first time in cells of the marine mollusk Onchidium verruculatum and identified it as a K+ current. The activation and inactivation of the current produces a characteristic “A” profile, which is the reason for the name 23. The equilibrium potential of IA is similar to that of the delayed rectifier and is activated with hyperpolarization. The capacity of IA to trigger action potentials and excitability in various neurons has been extensively studied. The function of IA in the AP train is to decrease the firing frequency 24. Gustafsson et al. 25 discovered the existence of IA in CA3 neurons and found it to be decreased by 4-amonopyridine (4-AP), a convulsant, thus resulting in a marked increase in cellular excitability. In rat upper cervical ganglion neurons, IA was characterized as being very rapidly activated at -60 mV potential, depending on the concentration of external K+. This activation was reduced with 4-AP 26. In neostriatal neurons, Bargas et al. 27 demonstrated that IA is responsible for the delayed appearance of the action potential in response to near threshold depolarizing currents.
IA has been simulated with the aid of specialized programming languages for neuroscience. For example, it was simulated on a typical laterodorsal tegmental neuron with NEURON software 28. To examine the role of IA in excitability, network synchronization and epilepsy, Fransén and Tigerholm adopted a modified version of the model by Migliore et al., downloaded from the ModelDB 29,30,31. However, there is no education simulator, to our knowledge, for IA (http://sense- lab.med.yale.edu/senselab/modeldb/).
The relevance of IA is evidenced by its participation in several regulatory mechanisms in neurons. The electrophysiological data reported depends on the recording techniques involved 32.
The aim of the present study was to generate educational software to facilitate the teaching and learning of IA current in undergraduate and graduate programs. A series of simulators were grouped into three computer programs: IA Current, IA Constant-V Curves and IA AP Train. With electrophysiological techniques, they reproduce IA and allow for an unlimited number of virtual experiments.
Materials and methods
Three interactive computer programs were designed and developed to study IA: IA Current, IA Constant-V Curves and IA AP Train. For this purpose, the Visual Basic 6.0 programming language for Windows® environment was adopted. The programs were compiled and made executable for Windows, from Windows 7 to Windows 10.
The programs act as simulators. To simulate a neuron, the Hodgkin-Huxley model 9,10,11,12,13,14,15 was employed (equations 1 and 2) and IA was added in accordance with the model described by Connor et al. 24 (equations 3, 4, 5, 6 and 7).
Where Cm is the membrane capacity per unit area (assumed constant), V is the membrane voltage, and gNa, gK, gA and gL are Na+, K+, IA and leakage conductance, respectively. ENa, EK, EA and EL are the equilibrium potentials for Na+, K+, IA and leakage, respectively. The equations utilized for Na+, K+ and leakage conductance and the corresponding velocities were those proposed by Hodgkin and Huxley 10, (see Hodgkin-Huxley equations for INa, IK and IL in Cronin 33 and Sterratt et at. 34).
The equations for IA are 24,34:
Where GA is the maximum conductance of IA, a is the activating particle and b is the inactivating particle 34.
The kinetics of the activation curves a∞(V) and b∞ (V) (steady state) and time constants (a(V) and (b(V) are:
The parameters used in the model are: Cm = 1 μF cm-2; ENa, EK, EA and EL = 50, -77, -80 and -22 mV, respectively; gNa, gK and gL = 120, 20 and 0.3 mS cm-2, respectively; gA is variable, being selected by the simulators.
IA Current program
The IA Current program was designed to generate macroscopic traces for IA during the voltage clamp technique. It is comprised of four simulators: (1) IA Current Traces, (2) IA Inactivation Protocol, (3) IA Equilibrium Potential and (4) IA Activation-Inactivation Plots.
The IA Current Traces simulator, based on the mathematical model reported by Connor and Stevens 24,35,36,37, reproduces the outward currents (IA).
The IA Equilibrium Potential simulator, also based on the Connor-Stevens model, examines the equilibrium potential for IA.
The IA Inactivation Protocol simulator involves adjusting the voltage holding (VH) to lower and lower negative potential levels before applying the stimulus pulse (voltage command, Vc). The kinetics of the decrease in the peak was derived with a mathematical fit from the experimental data of Connor and Stevens 35,36,37.
The IA Activation-Inactivation Plots simulator consists of mathematical equations describing the rate constants 35 ,36,37, which were also obtained from the experimental data of Connor and Stevens. By employing these equations, the activation and inactivation currents of IA can be portrayed.
IA Constant-V Curves program
Based on the Hodgkin-Huxley model 10, this program replicates the voltage dependence of the speed constants.
IA AP Train program
This program is designed to reproduce the action potentials of neurons with an inward sodium current (INa), an outward K+ current (IK) that does not inactivate (H-H type, Hodgkin and Huxley 10), and an outward potassium current that inactivates (IA) (equation 1).
To start the integration, a fourth-order Runge-Kutta method with a step time of dt = 0.01 was used. An algorithm written in basic to solve differential equations with this method is found in Zill 38.
Figure 1 illustrates the flow chart for the implementation of the models in Visual Basic®.

Figure 1 Flow chart for implementing the models. For each simulator, the values of the variables and the stimulus pulse are entered. The program initializes the graph scales and the basal values of the model. The differential equations are solved simultaneously, and the results are presented graphically. The iteration time depends on the duration of the stimulus (dp). The user can exit the simulator at any time.
Results and discussion
Three interactive computer programs (IA Current, IA Constant-V Curves and IA AP Train) were designed and developed to reproduce the electrophysiology of IA.
IA Current program
A-type potassium current simulations
The user interface of the IA Current simulator has two oscilloscope screens. One shows the macroscopic current traces of the IA and the other illustrates the stimulus pulse in its voltage clamp mode.
The stimulus voltage to perform the simulations is in the range of -60 to -20 mV, with VH = -93 mV. In the neurons recorded by Connor and Stevens 36, the activation time constant has values from 10-25 ms and the inactivation constant from 220-600 ms. These values vary according to the type of cell. In neurons of the hippocampus of the vertebrate central nervous system, the values are considerably lower. Experimental values from other neurons can also be employed. To simulate and replicate the experimental results in distinct neuronal systems, the user need only enter the time constants of different neurons.
The simulation of the experiments published by Connor and Stevens 35,36 is depicted in Figure 2.

Figure 2 The simulation of the experiments carried out by Connor and Stevens were run to study the IA. The voltage setting pulses are displayed on the lower oscilloscope screen. The upper screen portrays the currents traces corresponding to Vc = -60, -50, -40, -30 and -20 mV (the curves from baseline upwards). The VH was -93 mV and the rise time and decay current constants were 25 and 300 ms, respectively.
Command voltage pulses of -60, -50, -40 and -20 mV were applied. As the command voltage becomes less negative, the amplitude of the current increases 36. The IA trace corresponding to each of the stimulus pulses is displayed on the upper oscilloscope screen. For each simulation, the value entered for the time constant was 25 ms for activation and 300 ms for inactivation.
The simulations generate a classic IA profile. This is a K+ output current that starts at hyperpolarized potentials and shows an ascending curve during activation until reaching a maximum, at which point it gradually descends during inactivation. With a new command voltage and VH, there are changes in the channel conductance (gA) and consequently in the current and peak amplitude of the current. The data correspond to experiments carried out at 5 °C. The currents are very slow in gastropod neurons compared to those in the brain of rats or other vertebrates. IA is presented directly without considering the total outward potassium current and IK.
IA conductance (gA) simulations
The channel conductance (gA) is obtained by dividing IA by the voltage command (Vc). Figure 3 shows the same simulation conditions as Figure 2. The upper oscilloscope screen depicts the gA value in nS cm-2. The trace duration is 1 s.
Simulation for IA equilibrium potential
One of the methods to determine the equilibrium potential of an ionic current is to perform voltage clamp experiments and find the command voltage where the current is zero. In the IA Equilibrium Potential simulator, the equilibrium for the current was -63 mV (Figure 4). The current-voltage relation is not presented.
Inactivation protocol simulation
To simulate the inactivation protocol, the IA Inactivation Protocol program is provided with the interface depicted in Figure 5. The two oscilloscope screens on the left side show the current traces of IA (upper) and the stimulation pulses (lower). The macroscopic IA current is recorded in a neuron, beginning with a test depolarization at 0 mV and followed by the holding potential that varied from -90 to -50 mV.

Figure 5 Simulations with the inactivation protocol. The voltage holding (VH) was applied at -80, -70, -60, -50 and -40 mV, consecutively. The responses are displayed on the upper oscilloscope screen. The amplitude of the trace was greatest at -80 mV and least (2.6 nA) at -50 mV.
As can be appreciated, the peak value of the current decreases as the holding voltage preceding the stimulus pulse becomes less negative. Figure 6 illustrates how the decline in the peak value follows the pattern of a decreasing curve, as reported by Connor and Stevens 36. The maximum value of IA was obtained with a holding potential of -90 mV, and a value near zero was found in the range of -50 to -40 mV.
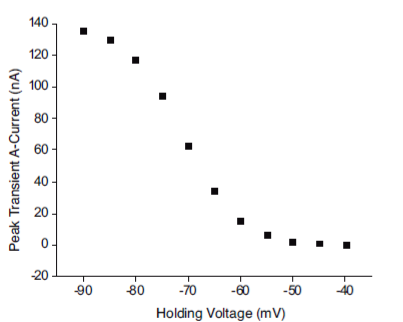
Figure 6 Inactivation plot. The peak value of the current decreases as the VH becomes less negative, from -90 to -40, in decremental steps of 5 mV. These results replicate the experimental data 36.
Simulation of the activation and inactivation curves
Simulations of the activation and inactivation curves are based on the Hodgkin-Huxley model 10. Figure 7 shows an example of simulation. Curves a(V, ∞) and b(V, ∞) overlap between -30 and -20 mV.

Figure 7 Simulation of activation and inactivation curves for the IA. The decreasing curve corresponds to inactivation and the increasing curve to activation. As the membrane potential becomes less negative, its activation is greater. The simulation is in agreement with the results of Connor et al. 37.
IA Constant-V Curves program
This program allows the user to observe the voltage dependence of the opening and closing speed constants for IA. In the first simulation, the values entered were those reported for gastropod cells (Figure 8).
IA AP Train program
The Train IA program simulates a neuron with the sum of the currents IA, INa and IK. It reproduces action potentials when the neuron is stimulated by a current pulse lasting from 50-200 ms at an intensity of 8 nA cm-2. A test simulation to study the effect of IA on the AP train is shown in Figure 9.

Figure 9 Simulation of the effect of IA on the AP train. With gA = 50 mS cm-2, it is clearly observed how this current hyperpolarizes the neuron and decreases the AP frequency (39). Control action potentials (utilizing Na+ and K+ channels) in black, IA action potentials in red.
Two simulations were performed in a row with gA set at 50 mS cm-2. In the first, the AP was generated in a neuron with only Na+ and K+ channels (trace in black).
In the second, the AP was triggered in a neuron that also has A-type potassium channels (red line). Note how the time between APs increases in the second. Hence, IA delays the appearance of the AP and the frequency of the AP train decreases.
The overall model
A-type potassium channels are found in the neurons of mollusk and of the central and peripheral nervous system of mammals. The physiological implications of this type of channel reveal its importance for the appropriate functioning of neurons and the brain as a whole.
Programs for simulating IA, such as NEURON and GENESIS, are freely accessible (https://www.neuron.yale.edu/neuron/download; http://genesis-sim.org/). For their proper use, however, it is necessary for the operator to undergo specialized training. Moreover, a high-speed, high-performance computer may be required, depending on the number of compartments simulated and the complexity of the model. Examples of special equipment with these specifications are a workstation or parallel supercomputer system.
Simulations of IA have been carried out for research purposes. For example, Huguenard and McCornick 40 reported simulating IA in the rhythmic oscillation of thalamic neurons.
Nevertheless, the tools employed imply considerable drawbacks for teaching purposes. They are very large and a substantial investment of time is required to learn how to manage them 15.
Due to the pandemic affecting us today and in the near future, online education has assumed increasing importance. The development of simulators with limited extension facilitates their usage, handling and transport. Furthermore, they can be easily adapted to remotely support teaching practices 15.
In the present work, a series of educational simulators were combined to reproduce the fundamental electrophysiology of IA under experimental conditions of current and voltage clamp. The simulators were validated with the mathematical models developed by the experiments of Connor and Stevens 35,36,37. The programs that group the simulators are compatible with on any personal computer having the minimum characteristics to run Windows® 7 to Windows® 10.
Thus, the software package developed presently will allow students to perform the experiments of Connor et al. 35,36,37. The results and implications of IA in the electrophysiology of the neuron can then be discussed with the instructor. The mathematical model currently employed for IA was the one published by Connor et al. 24. A description of the mathematical models proposed for IA is found in Rush and Rinzel 39. The numerical solution of differential equations enabled the reproduction of reported experimental data. Operation of the programs does not require specialized training. The user need only rely on scientific articles or on an instructor for an explanation of the biophysics and electrophysiology of IA.
Since experiments involving organic tissue remain costly, despite efforts to decrease the cost of electrophysiological recording 32, simulators provide a practical alternative for study and research in neuroscience. They have the advantage of permitting changes in the biophysical factors of the neuron in order to observe their influence on neuronal functioning, a capacity not shared by experimental animal models. In the case of the model described herein, the simulators enable students and teachers to vary the conductance of the A-type potassium channel or the time constants for the generation and decline of IA.
The development of simulators requires validation with experimental data. In regard to the simulators developed in the current investigation, real experimental values were entered for the variables, finding that the model reproduced the experimental results. Hence, the variables can be modified to analyze their consequences in a reliable model.
Conclusions
The three interactive computer programs described herein were able to replicate the biophysical characteristics of IA and provide a virtual reproduction of the electrophysiological processes involved in activating and inactivating an ion current.
The corresponding oscilloscope screens showed voltage-dependent curves. The IA AP Train program simulates the influence of IA on an AP train, revealing how it decreases the AP trip frequency in the trace. With this simulator, the effect can be observed of increasing or decreasing gA (channel conductance), modifying time constants, and altering the kinetics of the speed constants, among other phenomena. The simulators should be considered a teaching tool and do not replace the professor.











 nueva página del texto (beta)
nueva página del texto (beta)












