Introduction
Brain-Computer Interfaces (BCI) are systems that decode users’ intentions from the central nervous system in order to control external devices [1]. Applications for BCI include entertaining [2], neuromarketing [3], environmental control [4], communication [5], and treatment of motor-related diseases such as spinal cord injury and stroke [6]) ([7]. BCI were initially conceived for the later application, since they can provide an external communication pathway between motor impaired users and assistive devices. Particularly, during the last 10 years, BCI interventions have been studied as therapies aimed to improve upper limb motor recovery of stroke patients [8]. One of the main reasons for the growing interest in BCI for stroke rehabilitation is that patients can still control a BCI despite damaged brain tissue. In addition, since stroke is one of the main causes of motor disability worldwide, the research of new therapies is a priority in order to decrease the burden in healthcare systems [9].
For these reasons, several research groups have focused in the development of BCI technology, and its assessment for upper limb rehabilitation of stroke patients. However, a consensus regarding the efficacy of BCI for stroke rehabilitation has yet to be reached.
One of the reasons for this is that different variables related to the experimental design of reported studies, could affect rehabilitation outcomes of the patients. Therefore, this study aims to provide a comprehensive review of up-to-date literature related to BCI applied to upper limb motor rehabilitation of stroke patients, with the focus of analyzing study designs and outcomes. Although other reviews regarding BCI for neurorehabilitation have been reported [10] [11], to the authors’ knowledge a review that assesses these experimental design variables is still needed. This is addressed by first explaining theoretical fundaments, trends within the most important studies published and variables related to study design.
Stroke
Stroke is caused by a blockage (ischemic), or rupture (hemorrhagic) of blood vessels within the brain. This causes damage within the corticospinal tract of the affected hemisphere producing hemiparesis, paralysis of the body’s hemisphere contralateral to the lesion [12]. Stroke can also produce aphasia defined as a communication disorder acquired due to brain damage that affects the ability to understand, produce and use language [13]. Loss of vision and behavior changes can also be presented in stroke survivors [12]. Stroke sequalae is most likely caused by size and location of the lesion, making each patient’s stroke unique regarding the extent of the produced disability [12]. However, motor impairment produced by hemiparesis is one of the most significant sources of disability worldwide, with an estimated incidence of 795,000 new cases per year, and a prevalence in 2.5% of the United States population [9]. Stroke burden has mainly increased in low and middle-income countries [9], including Mexico, where incidence has been estimated in 339 new cases for each 100,000 inhabitants per year, with a prevalence of 18.2 for every 1000 people older than 60 years [14]. Therefore, approximately 200,000 people live with stroke sequalae in Mexico, and this number will rise as population with more than 60 years increases. The rise of stroke burden in global healthcare systems and, the low motor recovery rates of patients with severe initial motor impairment, highlight the importance of researching techniques for complementing conventional treatment for stroke.
Stroke Rehabilitation
After the initial symptoms of stroke, patients need urgent medical attention. This attention must be comprised by diagnosis through computed tomography (CT) or magnetic resonance (MR) images. After diagnosis confirmation, acute stroke management can include endovascular and intravenous thrombolytic therapies, and management of blood pressure, glucose, temperature and oxygen [15]. Once the patient’s condition is stable, treatment mainly relies in rehabilitation therapies. Some guidelines for stroke treatment include interdisciplinary approaches focusing in: dysphagia and nutritional management, upper and lower extremity rehabilitation, communication and cognitive enhancement therapies, and management of secondary complications [16]. Specifically, upper limb standard rehabilitation is focused in providing occupational and physical therapy during subacute (at most 1 year after the stroke onset) and chronic (more than a year after onset) stages of stroke [17]. Occupational therapy is comprised by repetitive, progressive and targeted specific oriented exercises, aimed at performing activities of daily living [16]. Physical therapy is comprised by the application of physical agents such as water and temperature for increasing upper limb function and decreasing excessive muscle tone caused by spasticity [16]. Even if standard rehabilitation is provided to stroke patients, it is estimated that only 35% of patients with severe initial impairment will recover enough upper limb motor function to use their paralyzed limb for daily activities [17]. Therefore, other therapies besides standard treatment have also been evaluated for upper limb motor rehabilitation. Some examples include mirror therapy, transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS), virtual reality and motor imagery. One of the therapies that has achieved enough evidence to be regarded as a promising complement to standard therapy, is rehabilitation using robotic assistive devices [18]. Upper limb robotic interventions have the advantage of increasing the number of therapies provided by healthcare systems, which number of standard sessions and session’s time per patient have been reduced due to the increasing number of patients requiring stroke treatment [19]. Several robotic systems such as the MIT MANUS [20], Amadeo [21] and ARMEO [22] have been developed, and their effects are still being reported in stroke patients’ upper limb rehabilitation. BCI are another potential therapy that can be coupled to robotic assistive devices for stroke rehabilitation. The main goal of all therapies, standard or experimental, is neural plasticity enhancement. Neural plasticity is the ability of the brain of reorganizing and is presented during learning and recovery after brain damage [17]. In order to measure patients’ upper limb disability, several scales are currently used in the clinical environment. Some of the most used are the Fugl-Meyer Assessment for the Upper Extremity (FMA-UE), which assigns a score between 0-66 points, with a higher score suggesting a lower impairment [23]. Another widely used clinical score is the Arm Research Arm Test (ARAT), this scale assigns a 0-57 score, als with a higher score suggesting lower upper limb impairment [24]. Unlike the FMA-UE, for the ARAT, special items are needed to perform the test, such as wooden blocks of specific dimensions and sizes. Both FMA-UE and ARAT have shown to have similar scores for assessing upper limb motor impairment in stroke patients, however, they are not regarded as equivalent [25]. Other clinical assessments of stroke upper limb impairment are the Stroke Impact Scale (SIS) [26], the 9-Hole Peg Test (9HPT) [19], European Stroke Scale score (ESS) [27], Goal Attainment Scaling (GAS) [28], Motor Activity Log (MAL) [29], Motor Assessment Scale (MAS) [30], Stroke Impairment Assessment set (SIAS) [31], Modified Barthel Index (MBI) [32], Medical Research Council muscle strength scoring system (MRC) [33], Jebsen Hand Function Test (JHFT) [34] and, the National Institutes of Health Stroke Scale (NIHSS) [35]. Physiological based evaluations have also been reported for assessment of upper limb hand disability, such as quantitative EEG [36], dynamometry, Transcranial Magnetic Stimulation (TMS) [37] and range of movement (ROM).
Brain-Computer Interfaces
BCI are systems that translate brain signals into commands to control external devices [1]. Brain signals can be acquired by means of different 207 techniques as magnetoencephalography (MEG) [38], near-infrared spectroscopy (NIRS) [39], electrocorticography (ECoG) [40], local field potentials (LFP) recordings [41], electroencephalography (EEG) [42], among others. MEG has the best compromise between time and spatial resolution, however, it also the most complex acquisition modality for BCI and the least available for practical applications. To the authors’ knowledge, in Mexico, no public health care institution has MEG devices. NIRS is a noninvasive technique but has poor time resolution. While ECoG and LFP are invasive techniques, it means that a neurosurgery needs to be performed to implant microelectrodes in the brain tissue. The disadvantage of these invasive methods is that the quality of the signal decreases in long-term recordings, making time and spatial resolution unfeasible for BCI applications in a matter of months [43]. EEG has a good time resolution, and poor spatial resolution compared to MEG and invasive acquisition methods. But since the electrodes are placed over scalp, it has a relatively low cost making it highly accessible and acceptable by patients. In almost all public healthcare institutions in Mexico there is a device for EEG recording. Hence, EEG is the most suitable technique to implement acquisition of brain signals in BCIs. In order to translate brain signals into control commands, a processing algorithm is required, comprised by preprocessing, feature extraction and classification of the signal. In the state-of-art literature, there are several methods that have been tested to process EEG signals for BCI [44] [45] [46]. Indeed, most published papers in the subject of BCI are related to new processing algorithms tested offline. But, the implementation of a close-loop BCI requires algorithms running in online applications. Another stage of BCI is feedback, which comprises external devices, this stage depends on the goal of the BCI and it can be divided in three different applications: communication, substitution and rehabilitation. In the first, the external device can be a computer monitor; in the second, a hand prosthetic or a wheelchair; and in the third, visual feedback displayed in a screen, a robotic hand orthosis, augmented virtual reality, or neuromuscular electrical stimulation (NMES).
Figure 1 shows a depiction of a BCI system’s stages. The objective of BCIs applied for upper limb rehabilitation is to excite the peripheral nervous system in order to facilitate neural plasticity [47]. This closed-loop communication allows users to control, arm, wrists or fingers, with their movement attempt (MA) or motor imagery (MI), even if they are paralyzed due to hemiparesis. Using MI, stroke patients can still elicit similar cortical activations as the ones observed due to movements, as demonstrated by studies that used high resolution imaging techniques [48] [49]. A motor Imagery Questionnaire has been proposed to indirectly measure a subject’s ability to perform MI (KVIQ) [50].
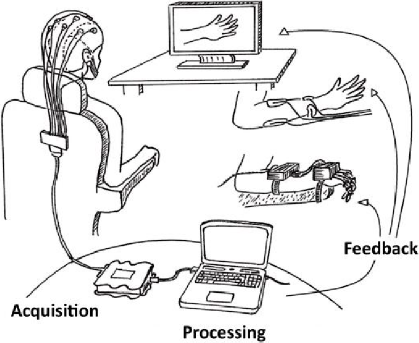
Figure 1 Stages of a BCI system. The three main types of feedbacks used for stroke patients’ upper limb rehabilitation are illustrated.
Here, a review of up-to-date literature regarding BCI applied for upper limb stroke rehabilitation was performed by classifying studies that used different types of BCI feedback into 3 categories: visual, robotic devices and NMES. The research was performed in PUBMED, Web of Science, Sciencedirect, Spingerlink, IOPscience, Taylor & Francis, Hindawi and IEEE Xplore, databases. In order for studies to be included in the current review, tests with feedback provided to patients had to be reported. The main objective of selected studies had to be the assessing of a close-loop BCI based on noninvasive techniques for upper limb stroke neurorehabilitation. Studies designs had to include a control group. Therefore, case studies and case series studies were not included, while clinical studies, and controlled pilot studies were included in the review.
BCI with robotic feedback
Therapies with robotic assistive devices have proven effective for stroke patients’ upper limb rehabilitation. Therefore, several research groups have combined these devices with BCI, allowing users to control an exoskeleton or orthosis which provide passive movement to the fingers, wrist or arm of patients using MI or MA. Reported robotic assistive devices combined with BCI for stroke rehabilitation vary largely in complexity and degrees of freedom. One of these device types are hand robotic orthosis that provide finger flexion and extension of stroke patients’ paralyzed fingers. Such devices were reported by Kasashima-Shindo et al. in a BCI intervention using a simple design with a motor-driven hand orthosis which extends patients’ paralyzed fingers [51]. Ramos-Murguialday et al. assessed the effects of a BCI coupled to hand robotic orthosis, which also provided finger flexion and extension passive movements, with an orthosis that was fixed to a larger structure [7]. Carino-Escobar et al. reported the preliminary results of the effects of an intervention with a 3D-printed robotic hand orthosis, that allowed stroke patients to grasp a baseball placed in front of them using their MA [52]. Orthosis or exoskeletons that provide passive movement to the wrist have also been combined with BCI for stroke rehabilitation assessment by Ang et al. using a robotic knob actuator, that allowed patients to receive passive movement simulating the opening of a door knob [53]. Other robotic devices provide movement to patients’ arms, such as the MIT-Manus robot exoskeleton. This device has been used as BCI feedback in several studies which assessed BCI interventions in stroke patients, for example, in the studies by Ang et al. [15] [54] and by Varkúti et al. [55]. Table 1 shows details of studies which BCI systems’ feedbacks were comprised by robotic assistive devices.
Table 1 Features and outcomes of the included studies for BCI for upper limb rehabilitation of stroke patients. (*) Preliminary results published in [52].
| Robotic Orthosis | |||||||
|---|---|---|---|---|---|---|---|
| Authors | Stroke Type |
Chronicity | BCI Group (n) |
Control Group (n) |
Intervention Duration |
Outcome Measure |
Improvement (FMA-UE) |
| Ramos- Murguialday et al., 2013 |
No data | Chronic | MI (16) | Sham (16) |
4 w (5 d/w, 60 min/d) |
FMA-UE, GAS, MAL, MAS |
ΔBCI=2.7 ΔControl=0.5 |
| Ono et al., 2014 |
No data | Chronic | MI (6) | Visual feedback | 12-20 d, 60 min/d | SIAS, EMG, qEEG |
No data |
| Ang, et al., 2014 |
Ischemic
and Hemorrhagic |
Chronic | MI (6) | Haptic knob (8) Standard therapy (7) |
6 w (3 d/w,90 min/d) |
FMA-UE | ΔBCI=7.2 ΔControl1=7.3 ΔControl2=4.9 |
| Ang, et al., 2015 |
Ischemic
and Hemorrhagic |
Chronic | MI (11) | MIT-Manus (14) |
4 w (4 d/w, 60 min/d) |
FMA-UE | ΔBCI=4.5 ΔControl=6.3 |
| Kasashima-Shindo et at., 2015 |
Ischemic
and Hemorrhagic |
Chronic | MI (7) | MI+tDCS (11) |
2 w (5 d/w, 45 min/d) |
FMA-UE | ΔBCI=6.6 ΔControl=6.0 |
| Frolov et al., 2017 |
Ischemic
and Hemorrhagic |
Subacute and chronic |
MI (55) | Sham (19) |
2 w (5 d/w, 30 min/d) |
FMA-UE, ARAT | ΔBCI=5.0 ΔControl=5.0 |
| Cantillo-Negrete et al., 2019* |
Ischemic | Subacute | MA (10) | Standard therapy (10) |
4 w (3 d/w, 30 min/d) |
FMA-UE,
ARAT, Dynamometry, TMS, qEEG |
ΔBCI=2.4 ΔControl=3.5 |
| Neuromuscular Electrical Stimulation (NMES) | |||||||
| Li et al., 2014 |
Ischemic
and Hemorrhagic |
Subacute | MI (7) | NMES only | 8 w (3 d/w, 60-90 min/d) |
FMA-UE, ARAT | ΔBCI=12.7 ΔControl=6.7 |
| Kim et al., 2016 |
Ischemic
and Hemorrhagic |
Chronic | MI (15) | Standard therapy (15) |
4 w (5 d/w, 30 min/d) |
FMA-UE, MAL, MBI, ROM |
ΔBCI=7.9 ΔControl=2.9 |
| Biasiucci et al., 2018 |
Ischemic
and Hemorrhagic |
Chronic | MA (14) | Sham (13) |
5 w (2 d/w, 60 min/d) |
FMA-UE, MRC, MAS, ESS |
ΔBCI=6.7 ΔControl=2.1 |
| Remsik et al., 2018 |
Ischemic
and Hemorrhagic |
Subacute and chronic |
MA (21) | No therapy (12) |
4-6
w (2-3d/w, 90 min/d) |
ARAT, SIS, 9HPT, Dynamometry |
No data |
| Visual Feedback | |||||||
| Mihara et al., 2013 |
Ischemic
and Hemorrhagic |
Subacute | NIRS-MI (10) | Sham | 2 w (3 d/w, 20 min/d) |
FMA-UE, ARAT, MAL, KVIQ-10 |
ΔBCI=5.0 ΔControl=2.3 |
| Rayegani et al., 2014 |
No data | Subacute | MI (10) | Standard Therapy (10) Standard Therapy+EMG (10) |
2 w (5 d/w, 60 min/d) |
JHFT | No data |
| Pichiorri et al., 2015 |
Ischemic
and Hemorrhagic |
Subacute | MI (14) | MI only (14) |
4 w (3 d/w, 30 min/d) |
FMA-UE, MRC, NIHSS |
ΔBCI=44.0 ΔControl=19.8* |
BCI with neuromuscular electrical
Stimulation Feedback
Similar to robotic assistive devices, neuromuscular electrical stimulation (NMES) has been evaluated by different research groups for stroke patients’ upper limb rehabilitation. For NMES application, surface or implanted electrodes over the muscle motor points or nerves that innervate targeted muscles are used with a stimulation frequency between 12 and 50 Hz. Strength of muscle contraction can be modulated by varying either pulse amplitude (0-100 mA) or width (0-300 μs) [56]. NMES can be applied in different muscles, depending on the number of channels, and type of movement targeted within a patients’ paralyzed upper limb. For example, BCI feedback reported by Kim et al. was comprised by the activation of wrist extensor muscles [57]. Another work by Biasiucci et al. used NMES to elicit finger and wrists extension [58]. The details of studies that apply NMES as BCI feedback for upper limb stroke rehabilitation can be observed in Table 1.
BCI with visual feedback
Some studies have reported upper limb motor recovery outcomes in stroke patients after a BCI intervention with exclusively visual feedbacks. Visual feedback has been hypothesized to have the potential of reinforcing motor learning by activating the mirror neuron system [59]. Visual feedbacks reported among studies are heterogeneous, for example, Mihara et al. reported a NIRS-based BCI that showed MI-related hemoglobin signals to patients by displaying them as a vertical bar in a computer monitor during BCI therapy [60]. Pichiorri et al. developed BCI provided visual feedback using a virtual hand displayed in the same location as patients’ actual hands [61]. A summarized description and outcomes of these studies is shown in Table 1.
Discussion
A total of 14 studies, including 202 patients, were identified in the literature complying with the inclusion criteria for this review [7] [51] [53] [54] [57] [58] [60] [61] [62] [63] [64] [65] [66] [67]. This number of patients is lower than the evidence reported in therapies recommended by the Union of European Medical Specialists Physical and Rehabilitation Medicine section for upper limb stroke rehabilitation, such as Constrained-Induced Movement Therapy (CMIT), mirror therapy, and robotic-assistive therapy [68]. There are 51 controlled trials and 1784 patients reported for CIMT [69], 15 studies which included 392 patients for mirror therapy [70], and 44 controlled trials encompassing 1362 patients for robotic assistive devices. Therefore, this implies that more studies which provide evidence of the effects of BCI therapies for stroke upper limb rehabilitation are still needed, in order to assess the feasibility of these systems and be included within clinical therapy guidelines.
After stratification of the revised studies based in the type of feedback, most of the studies reported the use of robotic assistive devices (n=7), followed by studies using NMES (n=4), and visual feedback (n=3), as shown in Figure 2B. The number of patients included in each subgroup also reflected this tendency with 111 patients included in studies with robot therapy, 57 with NMES, and 34 with visual feedback. An explanation for the higher number of studies and patients with robotic feedback could be that robotic devices have been regarded as safe and have potential for enhancing, albeit in a small degree, upper limb rehabilitation in stroke, as concluded by Bertani et al. [18]. On the other hand, NMES for upper limb stroke rehabilitation has been assessed in 8 trials encompassing 192 participants, and although a potential as a therapy was recognized, more studies are needed for confirming these observations [71]. Therefore, it could be suggested that robotic assistive devices are a more appealable choice for BCI feedback that NMES, at least until more evidence is provided. Visual feedback is the less used BCI feedback modality in the revised upper limb stroke studies. It is likely that the reason for this is that it has been reported that visual feedback produces less pronounced cortical activity in the motor cortex, compared to somatosensory stimulation, such as passive movement provided by robotic assistive devices and NMES [72]. Therefore, at the present time, BCI coupled to robotic assistive devices provide the highest evidence of the effects of BCI systems for upper limb stroke rehabilitation.
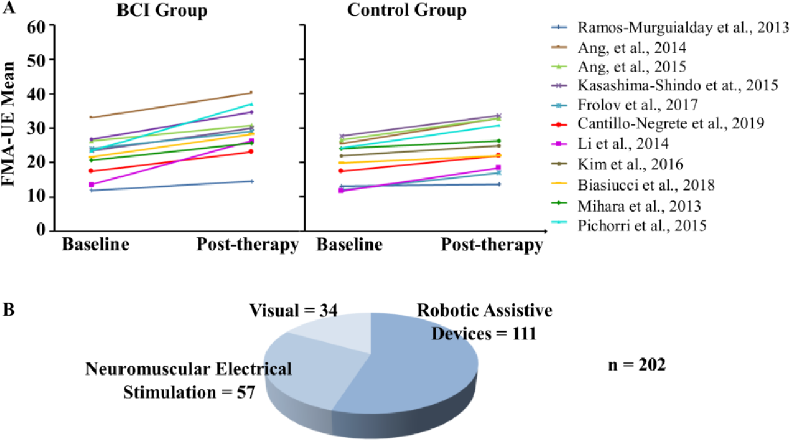
Figure 2 A) Baseline and post-therapy Fugl-Meyer Assessment for Upper Extremity measured in all the included studies. B) Number of stroke patients that have received upper limb BCI therapy for stroke classified by feedback type.
Baseline and post-therapy upper limb motor assessments of the groups of the included studies (Figure 2A), implied that all the experimental groups achieved in average an increase in upper limb motor function after the BCI intervention. Higher motor function was also observed in all except one of the control groups. In addition, as implied by the FMA-UE taken from Table 1, median recovery for all the studies was 6.6 points of the scale. Since the clinically minimal important difference has been set at a gain of 5.25 points of the FMA-UE [73], it can be inferred that a tendency towards a significant increase in upper limb motor recovery, is suggested by the reviewed literature. This provides evidence that patients are being benefited from their participation in studies aimed to evaluate the effectiveness of BCI for upper limb motor rehabilitation, even if they participate in the control groups of these studies. Therefore, stroke patient’s inclusion within these studies could increase their possibilities of achieving some degree of recovery. This could aid research groups that aim to assess BCI for upper limb rehabilitation to provide evidence that such intervention is likely to provide at least some motor recovery, and thus increase patients recruitment since low recruitment rates have been reported to reduce advancement in the field [53] [74].
Regarding differences between experimental and control groups, most studies reported higher recovery with the BCI intervention compared to the control group (n=7). The other reviewed studies reported lower mean recovery compared to controls (n=3), or the same mean recovery (n=1). However, only two of the studies that reported higher recovery with the BCI, stated that this recovery was statistically significantly higher compared to the control group [61] [63]. Therefore, the limited evidence that has been presented in the literature suggests that BCI interventions are likely comparable to other interventions such as therapy with robotic assistive devices and NMES for stroke upper limb rehabilitation. However, more studies are needed in order to assess if it is possible that a BCI intervention can be a more effective rehabilitation than other type of interventions.
Clinical characteristics of stroke within the revised studies, showed that most studies included patients with both ischemic and hemorrhagic stroke. This could increase the variability of the reported upper function outcomes, since it is known that hemorrhagic strokes present faster recovery rates compared to ischemic [75]. In addition, most of the reviewed studies (n=7) recruited stroke patients in the chronic stage of the disease, while fewer recruited subacute (n=5), and a mixed of subacute and chronic stroke patients (n=2). This is of relevance since it has been stated that neuroplasticity processes are more likely to be elicited during subacute compared to chronic stages of stroke [76], therefore, the effects of a BCI system in subacute and chronic stroke stages could be different. Therefore, a proposed strategy for reducing stroke patients’ upper limb rehabilitation outcomes variability in BCI studies, could be based in performing studies which only involve either ischemic or hemorrhagic, and either subacute or chronic stroke patients. Or alternatively, if the sample is large, then it is feasible to perform a separate analysis on the results from patients with different etiologies and time since stroke onset.
Intervention duration time could also affect motor upper limb outcomes among the reviewed studies, since although most of the experimental designs used a fixed intervention period (n=12), their duration was heterogenous. For example, the study with the highest intervention time was reported by Li et al. with an 8-week duration [63], while Kasashima-Shindo et al.[51], Frolov et al. [67], Mihara et al. [60], and Rayegani et al. interventions’ periods were of 2 weeks. Also, the number of BCI interventions for each week and duration of each therapy varied greatly across studies. Therefore, more evidence is still needed of the effects of BCI intervention time periods and motor recovery in stroke, since the relationship between treatment dosage and stroke recovery is still unknown 546 as stated by Cassidy et al. [77].
Finally, few of the reviewed studies complemented clinical assessments with physiological measurements such as qEEG (n=4). These measurements could aid to understand the neural plasticity mechanisms associated with upper limb motor recovery during an intervention with a BCI system. For example, several studies have provided evidence that cortical activations measured with qEEG above motor and non-motor regions, are associated with motor recovery [52] [78] [79] [80]. fMRI, an imaging modality with a greater spatial resolution compared to EEG, has also been used for hypothesizing a relationship between clearly identified cortical and subcortical regions with motor recovery [81]. Also, Transcranial Magnetic Stimulation (TMS) could be used to evaluate corticospinal tract integrity and cortical excitability of stroke patients during BCI interventions, since these variables have also been associated with motor recovery [82]. Therefore, if future studies include these physiological measurements, BCI for upper limb stroke rehabilitation, could be designed by taking into account how to increase neuroplasticity during the intervention.
Conclusions
Assessment of BCI for upper limb stroke rehabilitation has been reported in a low number of patients in order to draw conclusions regarding its effectiveness compared with standard or other experimental treatments. Furthermore, design features such as type of feedback, etiology of stroke and intervention duration, differ between studies. Therefore, a larger number of studies and patient engagement is still needed for assessing the clinical potential of BCI in upper limb stroke rehabilitation. Also, trends within the field were identified, such as the spearheading of robotic devices as feedback, MI or MA used as BCI paradigms, and that it is likely that patients achieve some degree of recovery during a BCI experimental therapy. In addition, increasing the coordination between research groups in order to reduce variability within studies designs and enhancing the engagement of areas with low participation in the research field, could aid in establishing the role of BCI systems for upper limb stroke rehabilitation. If the clinical role of BCI is established, then more and longer sessions of therapies could be provided for patients. In addition, since these systems could be automatized, less intervention of physiotherapists would be needed to provide therapies, which could aid to tackle the lack of personnel needed to treat stroke patients, expected by the WHO in the forthcoming decades [83]. Particularly, Mexico could be greatly benefited by a complementary therapy for upper limb stroke rehabilitation, which demand is also expected to increase as population ages.











 nueva página del texto (beta)
nueva página del texto (beta)


