Introduction
Periodic breathing (PB) is a breathing abnormality associated with various oscillatory forms characterized by rises and falls in ventilation, and Cheyne-Stokes respiration (CSR) is a more severe form of a PB pattern in which apneas and hypopneas alternate with repetitive gradual increases and subsequent gradual decreases in ventilation. Cheyne-Stokes respiration is one of several types of unusual breathing with recurrent apneas (dysrhythmias). This abnormal pattern of breathing can be seen in patients with hyponatremia, traumatic brain injuries and brain tumors [1].
PB has a prevalence as high as 70% in congestive heart failure (CHF) patients [2], and is associated with increased mortality [3], especially in CSR patients. Clinical studies show that elderly patients often have an altered breathing pattern, with PB and CSR, coinciding simultaneously with the presence or absence of CHF.
CSR is present in up to 40% of patients with CHF and several studies have shown an increased mortality in patients with both conditions [4-6]. Based upon small case series, patients with congestive heart failure and Cheyne-Stokes respiration have a significantly greater mortality [6,7], particularly if present during wakefulness [8] than those without Cheyne-Stokes respiration.
An effective respiratory pattern classification method as a clinical decision tool could provide patients undergoing treatment with a benefit in prognostic outcome and provide us with better tools to help patient recovery.
Efforts to characterize breathing patterns and classify patients using biological signals, mainly ECG and breathing rate, have already been developed using traditional time-domain analysis techniques. Garde et al [9] proposed two highly effective methods for distinguishing healthy patients from those with abnormal breathing patterns using both linear and non-linear classifiers with an accuracy level of 93% and 100% respectively. Another method to discern obstructive sleep apnea (OSA) from Cheyne-Stokes respiration (CSR) using frequency domain analysis from overnight electrocardiography (ECG) was described by Suhas, Vijendra, Burk, Lucas and Behbehani [10] yielding an 87.5% sensitivity and a specificity of 75%. Neural networks classifiers have also been applied to the detection of irregular breathing patterns [11].
While traditional methods might prove useful in patient classification, the characterizations of the cardiorespiratory interactions of the patients might help us better understand the mechanisms of respiratory regulation and the effect of cardiac regulation on abnormal breathing patterns. To better examine these interactions and their effect on the status of the patients a joint symbolic dynamics (JSD) analysis was applied to the cardiac (ECG) and respiratory (RESP) signals of the patients in this study.
The main idea behind the concept of JSD is the elimination of detailed information in order to keep the robust properties of the dynamics by a coarse graining of the measurement [12,13].
After patients’ cardiorespiratory profile characterization, a classification model based on statistical analysis was designed to discriminate between study groups.
Methodology
The cardiorespiratory profiles (electrocardiographic and respiratory flow signal) of 45 elderly patients (age 71+) with congestive heart failure were recorded for 15 minutes under no stress at the Departments of Intensive Care Unit at the Santa Creu i Sant Pau Hospital, Barcelona, Spain, and the Getafe Hospital, Getafe, Spain. The patients of the study (age 71-93) gave their informed consent to participate in the study and the recording was done according to protocols established by local ethics committees. Using clinical criteria based on the patients’ respiratory profile and medical observation, patients included in this study were classified into one of three study groups:
› Group 0, 20 patients (9 male, 11 female, aged 81.7 ± 8 years) that presented a non-periodic breathing pattern (nPB).
› Group 1, 17 patients (9 male, 8 female, aged 81.5 ± 7 years) that presented a periodic breathing pattern (PB),
› Group 2, 6 patients (2 male, 4 female, aged 81.3 ± 7 years) with a Cheyne-Stokes breathing pattern (CSR).
Patients with PB could or could not exhibit this behavior during the recordings.
Of the total 45 patients, two (patients ‘p0027’ and ‘p0042’) were removed from the study due to high noise in the signal recordings.
Both signals, ECG and respiratory flow signals (ECG and RESP respectively), were recorded synchronously with a sampling frequency of 250 Hz for 15 min. The patients’ ECG and respiratory flow signals were preprocessed to remove signal recording noise. Some of the signals showed some small drift and a simple correction algorithm based on drift compensation was used on the signals to minimize these deviations.
Signal processing and time series extraction
The cardiorespiratory signals for all 43 patients of the study were processed and analyzed using MATLAB® (v R2015) signal and data analysis software. Custom written computer software developed under MATLAB® was used to analyze both signals in order to extract the time series using an algorithm based on wavelet transform and analysis [14].
The original signals of the study were decomposed into a lower frequency signal using a discrete wavelet transform in order to remove noise and reduce signal details while preserving overall signal pattern to allow features to be more easily extracted. A biorthogonal Daubechies 4 (db4) wavelet was selected for signal decomposition.
The decomposed signals were automatically inspected for QRS complexes. By detecting these peaks in the down-sampled signal, the location of these peaks in the reconstructed signal had to be cross validated in the original ECG wave. The signal was then visually inspected and edited, if necessary. Ectopic beats were determined, removed and interpolated using an algorithm based on local variance estimation.
Respiratory flow signal was examined using an algorithm based on the zero-crossing of the respiratory flow signal. The respiratory flow signal was visually inspected and edited, if necessary.
After R peak and breathing episode detection on the original signals (ECG, RESP), a time series vector was then created from the intervals between R peaks and breathing episodes, as follows:
Where RR(k1) and Ttot(k2) represent the time series of the ECG and respiratory signals, respectively; and j1 and j2 represent the location of an R peak and a breathing episode in the original ECG and RESP signals, respectively.
Since heart rate and breathing rate greatly vary in both frequency and variability, most of the time series values do not correlate and both time series values had to be synchronized in order to correctly apply joint symbolic dynamics [12]. An interpolation of the original time series was made using an estimation method based on local values at a 250 Hz frequency (the sampling frequency for the original ECG and RESP signals).
This new RR(n) and T tot (n) time series allows us to compare and apply JSD to study the interactions between the two signals and their effect on the patient’s status.
A new bivariate vector x is obtained from the sampling of each value of the resampled time series, as follows:
From this synchronized sampled time series, x, a symbol sequence vector, named s, is obtained to look for symbols that might help us differentiate study groups.
Joint symbolic dynamics: feature extraction
After signal processing and analysis, the bivariate sampling vector for the time series was then transformed into a series of discrete symbols in order to extract information about the cardiorespiratory system and their relation to the patients’ respiratory pattern and clinical classification.
From the bivariate vector x (c,r), which represents the sample vector of the cardiac (c) time series RR (n) and the respiratory (r) time series
This new symbol vector contains information about the behavior of the system and its dynamics. Each of the vector components, cardiac (c) and respiratory (r) vectors, contains a total of n symbols, which are divided into words (wk) of length 3, with an overlap of τ (0, +1, +2) symbols. A raw 4 dimensional matrix for word occurrence was then calculated using the bivariate sampling vector s for all patients (third matrix dimension) across groups for each value of symbol overlapping (fourth matrix dimension, τ = 0, +1, +2). The first two dimensions of the matrix span an 8x8 matrix W raw (rows: c-cardiac word types; columns: r-respiratory word types), which ranges from word type [000,000]T to [111,111]T. The method for obtaining the occurrence matrix W and the symbol vector s from the time series x is illustrated in Figure 2.
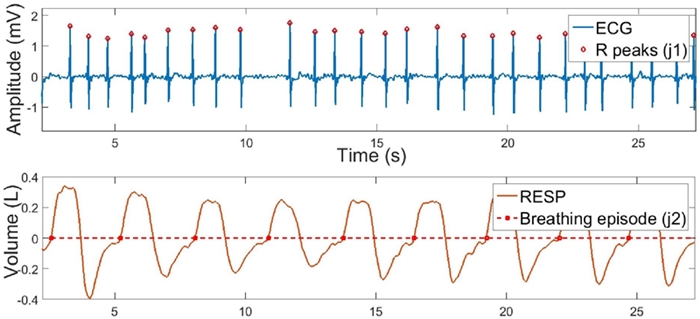
FIGURE 1 Original ECG and RESP signals after feature detection analysis. R peaks are shown in red diamonds above the ECG signal and breathing episodes are shown in red squares above the RESP signal.
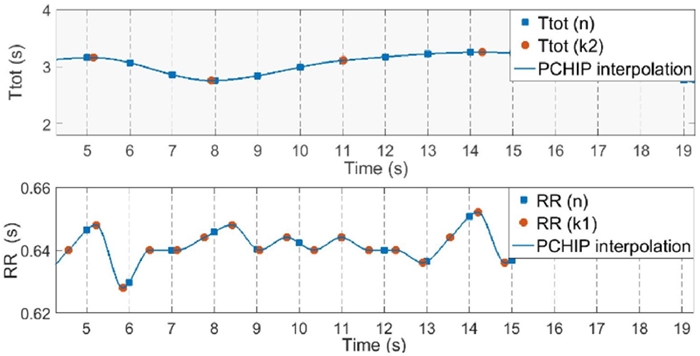
FIGURE 2 RR (n) and Ttot (n) time series calculated from the synchronized sampling of the interpolated original time series RR (k1) and Ttot (k2). Sampled values are shown in blue squares. Sampling frequency 1-Hz.
To compare word-type distributions between data sets of different length, the sum of all counted words for each group was normalized to 1. Word probability occurrence, p (W c,r) , was calculated separately for each symbol overlapping value (τ =0, +1, +2). The sum of each row in Wraw is computed as p (W c) and represents the occurrence probability of each word from the cardiac time series, as follows:
Where c takes binary values from “000” to “111”.
The sum of each column in Wraw is computed as p (W r ) and represents the occurrence probability of each word from the respiratory time series.
Where r takes binary values from “000” to “111”.
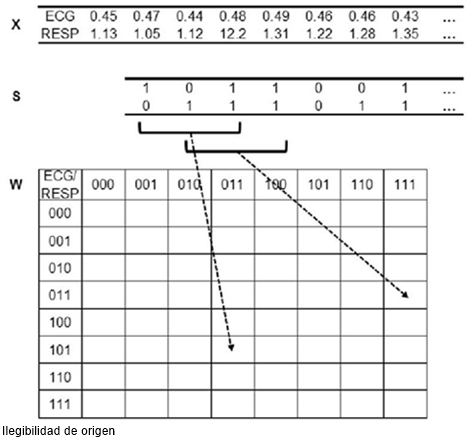
FIGURE 3 Process of joint symbolic dynamics (JSD). Transformation of the bivariate sample vector x (ECG = RR intervals [s]; RESP = respiratory episode interval [s]) into the bivariate symbol vector s (0: equal, decreasing or increasing values below 50% of the local standard deviation; 1: increasing values above 50% of the local standard deviation). Words share 2 symbols (overlapping value τ = +2).
Results
After the word occurrence matrix W was obtained, word probability distribution was plotted for each group to see if any significant difference across patient groups could be observed. A simple statistical analysis was then conducted on this matrix to obtain symbols with statistical significance across the groups. Single word types (cardiorespiratory words), as well as individual cardiac and respiratory symbols were examined across all groups to look for features that might prove valuable in patient classification. Individual words were then compared across groups to find those that were of statistical significance.
A Kruskal-Wallis test was performed for each individual parameter for parameter comparison across all groups. For parameter comparison between paired groups a simple Mann-Whitney U test was performed for each of the groups against the others.
Considering the problem of multiple testing, the necessary local significance level of a single parameter from an observed 64 dimensional parameter space had to fulfill Bonferroni’s inequality to guarantee a global significance.
Feature selection
Different discrimination functions were constructed using the most significant parameters from the symbolic analysis of the signals. The initial LDA model was created using the most prominent features from the statistical analysis yielding a 93.02% accuracy in patient classification. Word occurrence probability significance across groups was used to select words for the initial LDA classification model. Each parameter was selected only if it was present in at least two of three overlapping value distributions in order to reduce the possibility of overlapping dependent symbols.
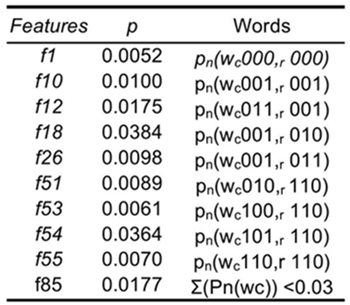
The best features to discriminate among groups are related to cardiorespiratory interactions or cardiac words alone (Table I). While the cardiac word for increasing intervals
Table 1 Words with significant probability occurrence across all three groups at an overlapping level τ = +2

Classification models were validated using a leave-one-out (LOO) cross-validation method. The best discriminant model was constructed using a stepwise feature selection to optimize accuracy from the initial model based on significant symbols from the Kruskal-Wallis test (Table II). This model used 10 features to discriminate among groups and yielded a 97.67% accuracy in patient classification. 9 of the 10 features selected are related to cardiorespiratory interaction, while the f85 parameter is the number of cardiac words
Discussion
JSD analysis revealed significant cardiorespiratory coupling patterns across the 3 study groups (non-periodic breathing, periodic breathing and Cheyne-Stokes breathing). Of these parameters, only one
The coupling of other physiologically relevant signals could potentially improve not only the classification model but also provide more information regarding the mechanisms involved in the poor respiratory regulation in PB and CSR patients. Patients with congestive heart failure and Cheyne-Stokes respiration have increased pulmonary vascular pressures and a JSD analysis using a blood pressure signal could potentially improve patient characterization. Patient characterization using JSD coupling ECG and blood pressure signals have already been developed providing good results [15-17].
Conclusions
In conclusion, by applying JSD we were able to provide detailed information about short-term regulatory mechanisms of respiratory patterns. From the results we can observe that the most relevant features selected prioritize cardiorespiratory variability rather than cardiac or respiratory variability alone, which supports the idea that abnormal breathing patterns originate from physiological abnormalities in chronic heart failure [18]. An adequate control of the cardiac status of a patient could prove a beneficial influence in CSR with improved sleep quality and quality of life for patients [19], and might help prevent the development of CSR in patients with normal breathing patterns.











 nova página do texto(beta)
nova página do texto(beta)