Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ingeniería biomédica
versión On-line ISSN 2395-9126versión impresa ISSN 0188-9532
Rev. mex. ing. bioméd vol.31 no.2 México dic. 2010
Artículo de investigación original
Radiofrequency based hyperthermia therapy: A centennial technique serving modern surgery
E.J. Berjano,* R. Romero-Méndez,** W. Franco***
* Department of Electronic Engineering, Universidad Politécnica de Valencia, Valencia, España.
** School of Engineering, Universidad Autónoma de San Luis Potosí, San Luis Potosí, SLP, Mexico.
*** Wellman Center for Photomedicine, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Correspondence:
Enrique J. Berjano.
Department of Electronic Engineering (Building 7F)
Universidad Politécnica de Valencia Camí
de Vera, 46022 Valencia, Spain E-mail: eberjano@eln.upv.es
Received article: 26/may/2010.
Accepted article: 30/november/2010.
ABSTRACT
Although the use of radiofrequency (RF) electric currents to treat very different illnesses by surgical means (e.g. cancer, disturbances in cardiac rhythms, or hyperopia) is very recent, the biophysical phenomena in which these surgical techniques are based has been understood for more than 100 years. The aim of this paper is to didactically present the use of RF currents to heat biological tissues with therapeutic and surgical purposes. First, the underlying biophysics mechanisms of this approach are explained. Next, different examples of recent clinical RF-current based applications are discussed.
Key words: Radiofrequency ablation, thermal therapy.
RESUMEN
El uso de las corrientes eléctricas de radiofrecuencia (RF) para tratar quirúrgicamente enfermedades tan diferentes como por ejemplo el cáncer, las alteraciones del ritmo cardiaco o la hipermetropía ocular es relativamente reciente. Sin embargo, el mecanismo biofísico en que se basan estas técnicas se conoce desde hace más de 100 años. Este artículo de divulgación tiene por objetivo presentar de forma didáctica y sencilla el uso de las corrientes de RF para producir calentamiento en tejidos biológicos con fines terapéuticos y quirúrgicos. En primer lugar se presentan las bases biofísicas en las que se sustenta dicha aplicación, y posteriormente se exponen diferentes ejemplos de aplicación clínica actual de las corrientes de RF.
Palabras clave: ablación por radiofrecuencia, terapia térmica.
INTRODUCTION
The use of radiofrequency currents (RF) for surgical treatment of diseases such as cancer, cardiac arrhythmia, or hyperopia is relatively new. However, the biophysical mechanism in which these techniques are based has been known for more than a century. Maybe this is why these techniques have been named (or should we say renamed) with impressive names, such as RF tumor ablation, percutaneous RF cardiac ablation, or conductive keratoplasty. Behind these denominations stands a technique developed at the end of the XIX century, whose objective is to heat biological tissue using RF currents. The use of heat as a means of therapy is also an old technique. The objective of this review paper is to present in a simple manner how RF currents are used to heat biological tissue in therapy and surgery. This requires a description of the relevant underlying biophysical principles, namely, how the interaction between RF currents and the tissue is produced. Another objective is to provide different examples of current clinical applications of RF currents. Rather than provide a simple enumeration of different applications, the purpose is to emphasize similarities and differences among applications stressing the fact that, from a historical perspective, the use of RF currents is based on the same biophysical principles that gave birth to electrosurgery a century ago.
THE LONG HISTORY Of THE THERAPEUTIC USE OF HEAT
Heat therapy has been employed for thousands of years to purify and detoxify. The steam baths used by the antique Greeks, the temascali used by the Mesoamerican inhabitants, and the Chinese herbal baths are examples of this millennial practice. Physicians and shamans of old civilizations made use of heat for treatment of injuries difficult to treat by other means. For instance, the ancient Egyptian medical papyrus found by Edwin Smith, dated 3000 BC, describes the use of cautery for treatment of ulcers and breast tumors1. In the millennial Indian culture, heated bars were used to stop bleeding2. The principles of cauterization for bleed arrest were known by Hippocrates (460-377 BC), who stated «those diseases which medicines do not cure, iron cures (reference to scalpel); those which iron cannot cure, fire cures; and those which fire cannot cure, are to be reckoned wholly incurable». Later on, the techniques for heat treatment were applied and improved by the Moors in the Iberian Peninsula. All these techniques applied the same concept: to place on the tissue to be treated an object previously heated (cautery), and wait until the tissue raised its temperature by thermal conduction. One big problem with all the procedures used at that time was the extraordinary pain that the direct application of heat produces on the nervous system.
A breakthrough in tissue heating technologies occurred in 1891, when d'Arsonval developed experiments on circulation of high frequency currents through body organs, observing that the currents did not produce any kind of nervous or muscular stimulation (and for that reason no pain or any other adverse effects), producing only a gradual heating3. Obviously, to make the currents circulate through the body, it was necessary the placement, in contact with the tissue, of metallic elements, known as electrodes, to close the electric circuit. Apart from the use of the electrodes (that could be similar to cauteries), the novelty and most important fact of this finding was that biological tissue could be heated directly by inducing an electric current, and not by thermal conduction from a previously heated element put in contact with the tissue. As a matter of fact, this experiment was the starting point of current electrosurgery, which uses electric currents of an approximately sinusoidal waveform with frequencies between 300 kHz and 1 MHz. The currents applied within this range of frequencies are the so called radiofrequency currents.
Two decades after the experiment of d'Arsonval, the German physician Carl Franz Nagelschmidt began to circulate high frequency electric currents within biological tissue, naming the procedure as «diathermy»4. Other important applications of electrocautery and diathermy focused in the treatment of lesions and malignant growths5-7. The first electrocautery device, known as the Bovie knife, was developed by Harvey Cushing and William T. Bovie in 19288, and was designed to stop bleeding of injuries by circulation of alternating currents in the radiofrequency range between an electrode in the form of a knife and a dispersive electrode of large dimensions located at a sufficient distance from the first. This development was of great importance, despite the fact that there were many methods to stop bleeding there were still many cases of patients considered not viable for surgery because of the risk of excessive bleeding. More than 80 years after its development, this device is still an essential tool in the surgical practice. This is how in the early 20th century, the first apparatus for application of RF currents in the medical practice were devised. The first designs were based on elemental electronic circuits made with capacitors and electric coils1. Later on, came the generators built with vacuum valves, and afterwards, with silicon transistors. These electrosurgical units allowed the operators to apply RF currents in a continuous manner, unlike the previous units that only allowed the application of brief pulses. This technology has changed little in our current surgical practice, for biological tissue cut with a certain degree of hemostasis (cut, coagulation and a combination of cut-coagulation), coagulation of small diameter blood vessels with bipolar clamps, or carbonization of surface tissue (fulguration). All these techniques are commonly used nowadays in the operating room.
INTERACTION OF RF CURRENTS AND BIOLOGICAL TISSUE
Firstly, we must remember the mechanisms by which some cells can be stimulated. In general, any electrical current is maintained by the movement of electric charge carriers. In the case of metals, electrons are the carriers of electric charge (each electron carries 1.6x10-19 Coulombs). However, inside biological tissue, ions in solution are the carriers of electric currents. In fact, in electrosurgery, the interface between tissue and electrode is where the charge is transferred between these two types of carriers (from electrons to ions or vice versa). Let us consider a simplified biological tissue structure made of cells surrounded by an extracellular medium (Figure 1). If we put two metallic electrodes in contact with the tissue and apply a voltage potential, for instance, with a battery, ions are carried towards the electrode of opposite sign (positive ions such as sodium Na+ towards the negative electrode, and negative ions such as chloride Cl- towards the positive electrode). We must remember that the cellular membrane is, in general, impermeable to the crossing of ions, reason for which the voltage differential produces an accumulation (reordering) of electric charge around the membrane or, to put it in other terms, a variation of potential across the cellular membrane. If this variation is beyond a threshold value, a series of phenomena are produced, causing the stimulation of the cell. In the case of muscle cells, such as cardiac cells, stimulation implies a mechanical contraction of the cell, and the coordinated contraction of all cardiac cells produce the heartbeat.
In order to produce a variation of potential across the cellular membrane above the threshold value, it is necessary to maintain the voltage differential between electrodes for a sufficiently long period of time. In the case of RF currents, an alternating current power supply is used (for instance a frequency of 500kHz) instead of a battery. This produces a signal with a voltage that varies with time in a sinusoidal manner, in which the polarity of the electrodes changes very rapidly, specifically at half the period of the signal, namely every millisecond. This time is sufficiently short to avoid a tension across the membrane above the threshold value. In the end, this motion back and forth of the ions produces the heating of the tissue by dissipation of the energy associated with the movement of ions (at the macroscopic scale this energy dissipation is known as friction). This is the reason why RF currents (between 300 kHz and 1 MHz) produce heating by ionic agitation without any kind of stimulation.
Another way of undestanding the interaction between RF currents and biological tissues is to consider the behavior of the cellular membrane as an electric capacitor, that allows the flow of RF currents without any resistance (as a matter of fact impedance), acting as a short circuit from the electrical point of view. This short circuit implies zero voltage across the membrane, that is, no possibility of alteration of the potential across, therefore no stimulation of the cell.
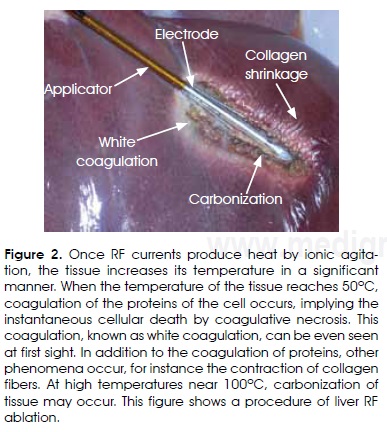
The heating produced by ionic stirring makes the tissue respond in a relatively rapid manner, and the tissue can reach 60°C in a few seconds, even though this depends on the applied electric power. When the temperature of the tissue reaches 50°C, coagulation of the proteins of the cell occurs, implying the instantaneous cellular death by coagulative necrosis. This coagulation, known as white coagulation, can be even seen at first sight (Figure 2).
The cells that are close to the 50°C isotherm and do not experience the lethal thermal dosage, but temperatures between 40 and 50°C, could develop a process known as apoptosis, and therefore die in a late and controlled manner. In addition to the coagulation of proteins, other phenomena occur, for instance the contraction of collagen fibers. Collagen fibers are macromolecules that form part of the mechanical structure of extracellular space. When the temperature of the tissue is raised, these fibers shrink in an important and irreversible manner.
When the temperature exceeds 80°C, other phenomena, such as desiccation (water loss), vaporization and even carbonization, may happen. It is important to remember that the RF currents used in electrosurgery are energy getting to the tissue by conductive mode, hence, two metallic electrodes are required to close the circuit for which the resistance is the biological tissue itself. The use of higher frequencies, such as microwaves (where the frequencies go from 915 MHz and 2.45 GHz), allows deposition of energy and total tissue absorption by means of irradiative phenomena, without electrical conduction. This is achieved by a surgical applicator that functions as a small antenna. In either case (microwave or RF), the interaction between energy and biological tissue produces the same effect and both belong to the group of non-ionizing radiations9.
THE LAST 25 YEARS
Since the mid 80s, RF currents as we know them, have beeen used in other less conventional applications, and in fact, in some cases, gave birth to other minimally invasive procedures that eventually replaced other complicated and risky surgical procedures. This does not mean that this type of energy has no further use in conventional electrosurgery, as in the case of coagulation of small blood vessels, on the contrary, this is a technique employed by any general surgeon in practically any kind of surgical intervention. However, modern applications show a very promising future. This is why this article highlights the advantages and disadvantages of some of them.
In general, in the context of the medical devices used to allocate RF current within the tissue, we have adopted the term «applicator» to designate an apparatus that includes one or more electrodes, being them metallic elements that are in touch with the biological tissue, from which the currents are distributed within the tissue. This terminology is in agreement with that proposed by the International Working Group on Image-Guided Tumor Ablation10.
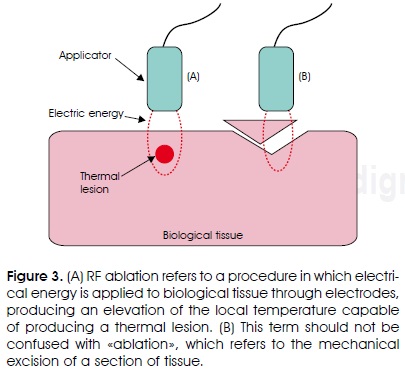
Some of the surgical procedures that use RF currents have been termed as RF ablation procedures, such as the ablation of cardiac tissue or tumors. In this sense, it is necessary here to contextualize the use of the term ablation. From a general perspective, ablation is the mechanical excision of a section of tissue. In the context of the high temperature thermal therapies, the ablation does not imply the excision or mechanical elimination of any part of the tissue, but the almost instantaneous physiological alteration of that part of the tissue by means of an important increase of temperature (Figure 3). We could consider that the ablation implies the «ablation of the original physiological function of the tissue»; i.e., tissue necrosis. This does not mean that the excision of tissue by means of electromagnetic energy is not possible, but very high energy sources are required to this end, as is the case of ultraviolet laser irradiation (excimer laser), which do cause the direct rupture of molecular links.
Radiofrequency cardiac ablation
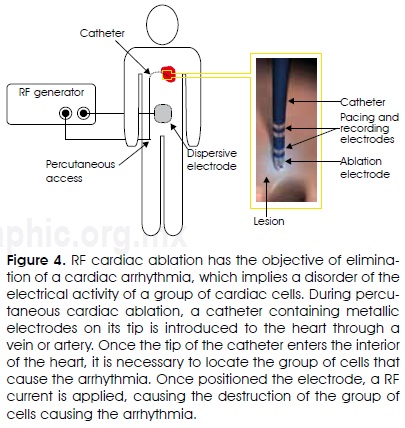
The objective of RF cardiac ablation is the elimination of a cardiac arrhythmia11. An arrhythmia is an electrical activity malfunction of a group of cardiac cells. The proper electrical activity of the cells originates the pacing of the heart and, therefore, the pumping of blood to all the body. During percutaneous cardiac ablation, a catheter containing metallic electrodes on its tip is introduced to the heart through a vein or artery (for instance the femoral vein). Once the tip of the catheter enters the interior of the heart (atrium or ventricle), it is necessary to locate the group of cells that cause the arrhythmia. This is achieved by means of an electrophysiologic mapping based on electric stimulation sequences with the simultaneous recording of the cardiac cells electric activity. The stimulation and recording are made with specific electrodes incorporated to the catheter. Once the group of cells to be eliminated (ablated) is located, RF currents are applied through the ablative electrode (which could be the same to the one used for cell stimulation or cell activity recording) and an electrode of large surface attached to the back of the patient (Figure 4). The maximal electrical current density in the tissue is located maximal at just under the ablative electrode, producing a localized raise of temperature at that zone, and therefore causing the destruction of the malfunctioning cells that originate the arrhythmia. Nowadays, some arrhythmias are treated using this procedure, giving other option to avoid open heart surgery, a much more complex and risky surgical procedure. In general, ablative cardiac procedures have used catheter coupled electrodes from 7Fr to 10Fr of diameter. Ablation electrodes usually have a length between 4 and 10 mm and a semispherical tip. For instance, for the ablation of accessory pathways12 conventional 7Fr and 4 mm electrodes are used, while for elimination of the auricular flutter the isthmus between the inferior vena cava and the tricuspid valve is ablated using a longer electrode13. In other cases, a complex pattern of lesions in the atria is produced in order to eliminate atrial fibrillation14, by repositioning the same electrode in several places.
So far, we have only considered percutaneous cardiac ablation, namely, that produced by means of a catheter. Cardiac arrhythmias can also be ablated through electrodes included in hand devices used by the surgeon during an open-chest surgery, or through small incisions (mini-thoracoscopy)15,16. In this surgical scenario, the ablation is made without heart arrest, namely producing lesions on the surface of the epicardium, or with heart arrest, using a system of extracorporeal circulation, and producing lesions to the internal surface of the heart (endocardium). In almost all cases of RF cardiac ablation, the power is modulated to maintain constant temperature at the ablation electrode. This power distribution method is known as temperature controlled ablation and is used not only in cardiac ablation but also in other procedures that we will review afterwards.
In RF cardiac ablation, it is important to reach temperatures that destroy the cells causing the arrhythmia: temperatures above 50°C for one minute or more. This approximate value of lethal dosage (50°C during 60 seconds) is also valid for other biological tissues, as in the case of the thermal destruction of tumors.
RF ablation of tumors
Another relatively recent application of RF currents is the destruction of malign tumors, e.g. liver tumors (hepotocarcinomas and hepatic metastasis). In this case, even though the procedure is supposed to be minimally invasive compared to conventional surgery, the current limitations of RF ablation make the surgical excision (mechanical) of the tumor the preferred surgical procedure (i.e. the gold-standard). Notwithstanding, the future of this technique is promising in cases where the patient cannot be subject to major surgery, as a matter of fact, there are many cases in which RF is currently used to ablate almost any organ (liver17, kidney18, lung19, and bone20). The procedure is based on the introduction of an electrode inside the tumor. This is achieved by piercing the skin of the patient (percutaneous approach) and guiding by means of some sort of imaging technique, as ultrasound, introducing the applicator directly in the target organ. This can also be done both during a laparotomy, namely with the open patient and the organ exposed. The electrodes are needle like21 (Figure 2), although there are more complex applicators including expandable needles with the object of ablating tumors of larger size22, and others that combine the infusion of a hyper saline solution within the tissue during the ablation in order to increase the lesion size23. Once the electrode is placed in the center of the tumor, a RF current is applied until the temperature of the cells exceeds the critical temperature (around 50°C) for several minutes.
RF assisted resection
In the area of oncologic surgery, there is an application of RF currents developed recently that has been named RF assisted resection24. It is basically a device that uses RF currents to reduce as much as possible the bleeding associated with the excision of the part of an organ that contains a tumor. This is achieved by the coagulation of blood vessels by heating produced during RF currents circulation. Again history repeats itself, that is, the use of a centennial technology assisting the creation of new medical devices, to perform innovative medical procedures. As examples of innovative devices we could mention those based on internally refrigerated electrodes that incorporate a blade for cutting pre-coagulated tissue25, electrodes that infuse a saline solution in the surface of the coagulation zone26, or those based on needles capable of creating a barrier of coagulated tissue before cutting with a cold scalpel27,28 (Figure 5).
Cosmetic surgery applications
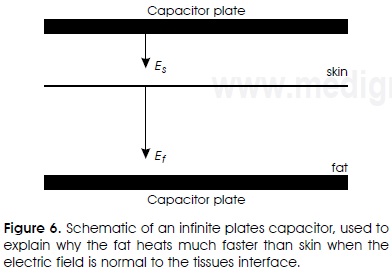
In the field of cosmetic surgery, there is now a strong push of technological development that aims at the use of RF currents as a non-invasive technique for selective heating of adipose tissue29,30. The objective is to destroy adipocytes, namely cells that contain fat, reducing in this way the fat of specific zones. The heating of fat is greater than that of skin when the internal electric field (E) is perpendicular to the interface of the tissue. This can be analytically demonstrated with an electrical capacitor of infinite plates as the one shown in Figure 6. The internal boundary condition should satisfy the following relation

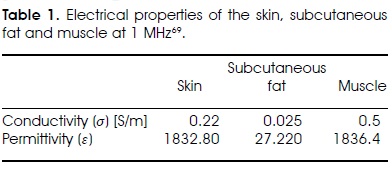
where ε is the complex relative permittivity. This relation is valid anywhere because the electric fields Es y Ef are constant in their respective tissues. The power absorption ratio per unit volume (Q) can be expressed in the following way
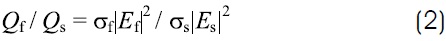
where σ is the electric conductivity. Combining relations 1 y 2, the ratio of power absorption can be written as a function of the electrical properties
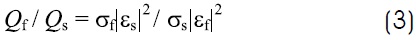
As an example, let us consider the application of a 1 MHz current (Table 1). In this case, the fatty tissue attenuates or dissipates less the electric field than the skin, σf /σs = 0.13. (Less attenuation means lower heat generation). However, the electric field of the fatty tissue is much more intense, Ef / Es = 83, consequently fat heating is much more intense than that of skin, Qf / Qs = 1029-31. A similar analysis shows that the fat heats much faster than the muscle, Qf / Qm = 20. In cosmetic surgery, this selective heating is advantageous. In other surgical procedures, such as breast cancer, it is not desirable to heat the adipose tissue.
When the electric field is not normal to the tissue surfaces, the opposite occurs, the skin heats much more than the fat. In that case the heat is used to contract collagen fibers, stimulating at the same time the generation of new collagen inside the dermis, causing as a consequence a softer and firmer skin, with a «younger» appearance.32. In this application is common to apply high levels of energy for short periods of time (of the order of milliseconds), because the objective is to reach very high temperatures to cause irreversible cellular damage. Unlike to what we stated before in the section on interactions of RF currents and tissue, these treatments are painful because the nerves that are sensible to temperature are located in the upper layers of the skin33,34. To minimize this problem, some devices cool the upper layer of the skin before and during the application of the RF current pulse, by using cold air, ice packs or cryogenic sprays. The idea is to minimize dermal damage, avoiding burns and mitigating pain. There are several types of applicators, but all of them use the same physical principles. The differences have more to do with geometry and number of electrodes, being the monopole (one electrode) and the bipolar (two electrodes) the most commonly used. A new concept is the fractional delivery of energy in order to promote a faster skin remodeling process . Within this concept an array of small non-invasive or minimally invasive (e.g., needle insertions) electrodes is used to deposit energy in small regions (focal points). As a consequence the damaged zones are microscopic in volume and surrounded by healthy tissue ready to start the healing process35-37. This concept is originally known as fractional photothermolysis, devised to accelerate the remodeling process of laser irradiated tissue38.
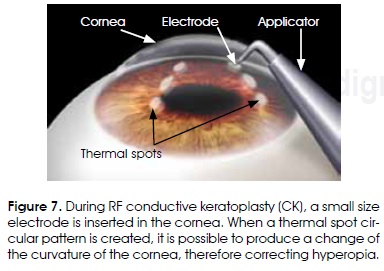
RF thermokeratoplasty
Other thermal applications of RF currents do not necessarily try to destroy tissue, but the irreversible contraction of collagen fibers. These applications have to do with very diverse surgical areas. For instance, RF currents are being applied for eye surgery, specifically in the cornea, using electrodes of very small size (Figure 7). The objective is to produce an irreversible contraction of the corneal stroma collagen, therefore producing a permanent change of its curvature39. The name of the procedure, in a broad sense, should be RF Thermokeratoplasty. When the lesions produced form a circular pattern of a sufficiently large diameter (6-8 mm), it is possible to produce pressure capable of elevating the central zone of the cornea, therefore correcting hyperopia by a procedure known as (conductive keratoplasty)40. Needle like electrodes of small thickness (90 /mm) and diameter (450 /tm) are used in this procedure. This procedure has also been used to treat astigmatism41 and keratoconus42.
Sphincter reshaping by RF
Other procedures in which collagen fibers are contracted try to correct the sphincters. Temperature controlled RF has been used to correct either the cardiac sphincter (union of esophagus and stomach) to treat gastric reflux43 or to treat fecal incontinence44. In the first case, a RF applicator is introduced through the mouth, to reach the union of esophagus and stomach. A RF current is applied producing a localized heating, capable of contracting the collagen fibers. This contraction narrows the union, reducing gastric reflux (Figure 8A)45. In the second procedure, a similar applicator is used, but in this case is introduced through the anus, after application of temperature controlled RF, the compliance of the tissue is modified, reducing considerably the symptoms of incontinency46.
Radiofrequency somnoplasty
Another application of collagen contraction is found in the treatment of obstructive sleep apnea47,48. The idea underneath this procedure is to contract collagen fibers of the tissue whose function is the obstruction (for instance the soft palate), and so to stretch the tissue that was excessively relaxed. Figure 8C shows an example in which a needle shaped electrode is applied in both the base and the muscles of the tongue. This procedure is known as somnoplasty. In this procedure, RF application is temperature controlled, being 85°C a typical programming value, while the power supply is stopped if the temperature goes beyond 105°C or falls below 65°C49, being this also an strategy in common use in RF percutaneous cardiac ablation.
Bronchial thermoplasty
Bronchial termokeratoplasty is based on ablation of the smooth muscles of the airways50,51, specifically affecting the capacity of structures to constrain, so reducing the frequency of asthmatic episodes and curing severe asthma in some patients52. In this system, a multiple electrode RF applicator is introduced through the mouth, expanding in the interior of the airways. Once in contact with the tissue, RF currents are applied in a controlled manner (Figure 8B).
RF based bipolar sealing systems
Although we have already described some of the novel procedures based on RF currents heating, we should not forget that the classical technique of bipolar electrodes blood vessel coagulation (Figure 9A) has experienced important technical progress in recent times. For instance, the equipment based on bipolar clamps has been further improved resulting in homeostasis systems adapted to specific procedures, which aimed at treating hemorrhoids53 the thyroid gland54 or the pancreatic tissue55. This devices are much more complex than the basic electrosurgical pincers, and could be disposable (Figure 9B) o reusable (Figure 9C).
Transurethral resection of the prostate
The modification of the electrodes traditionally used in electrosurgery has led to the development of somewhat complex devices, used to produce incisions of the prostate, of the neck of the bladder, or for resection of bladder tumors. These protocols are designed to increase the safety of the procedure56. Transurethral needle ablation (TUNA), is the name of a new technique that employs RF currents and electrodes modified from those used for other techniques. With this technique it is possible to accomplish the ablation of the prostate in patients with benign prostate hyperplasia57.
THE FUTURE
The investigation of new RF based hyperthermia therapies is focusing in three approaches: 1) to design applicators that permit to increase the efficacy and safety of the conventional techniques, allowing to have surgeries as minimally invasive as possible; 2) to develop imagingjechniques enabling to make thermal lesions in the right place, i.e. achieve higher spatial accuracy; and 3) to have systems that could give real time information of the lesion, both its geometry (dimension) and its characteristics (temperature values). In the years to come we will experience developments in these three complementary areas.
In relation to the development of safer and more efficient applicators, it is worth mentioning the miniaturization of the applicators and the constructive improvements, giving way to enhanced designs, for instance expandable systems. This means the electrodes would be positioned in the exact position, being very thin, and being introduced through very small orifices. Besides, it is important to highlight the experimental research efforts, aimed at combining RF currents (or of higher frequencies) with the use of nanoparticles, making possible to have a more circumscribed thermal effect, as in the case of ablation of tumors58,59.
Regarding the techniques to determine the right positioning of the lesions, the development has been especially intensive in the area of RF cardiac ablation60, even trying to eliminate the use of fluoroscopy61,62 by means of radio tracking. The development of noninvasive thermometry procedures is an area of promising future that has not still taken off63. Currently, imaging systems, such as ultrasound64 are being employed during the ablation of tumors, to make a real time follow up of the lesion evolution. Also, the techniques of imaging processing may improve significantly the outcome of existing surgery procedures65.
Finally, we should not forget the synergistic effects that some investigations have hinted may result from combining RF currents with other sources of thermal treatment, such as cryoablation66-68.
REFERENCES
1. Pearce JA. 1996, Electrosurgery, Chapman and Hall, London. [ Links ]
2. Radiofrequency ablation. National Institutes for Health Clinical Center web site. Available at: http://www.cc.nih.gov/drd/rfa/index.html. Accessed October 2010. [ Links ]
3. d'Arsonval MA. Action physiologique des courants alternatifs. CR Soc Biol 1891; 43: 283-286. [ Links ]
4. Licht S. «History of therapeutic heat», in Therapeutic heat and cold, S. Licht, Ed New Heaven, Conn., Licht., 1965; sec. 6: 196-231. [ Links ]
5. Beer E. Removal of neoplasms of the urinary bladder: a new method employing high frequency (oudin) currents through a cauterizing cystoscope. JAMA 1910; 54: 1768-1769. [ Links ]
6. Clark WL. Oscillatory desiccation in the treatment of accessible malignant growths and minor surgical conditions. J Adv Therap 1911; 29: 169-183. [ Links ]
7. Clark WL, Morgan JD, Asnia EJ. Electrothermic methods in treatment of neoplasms and other lesions with clinical and histological observations. Radiology 1924; 2: 233-246. [ Links ]
8. Cushing H, Bovie WT. Electro-surgery as an aid to the removal of intracranial tumors. Surg Gynecol Obstet 1928; 47: 751-784. [ Links ]
9. Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol. 2009; 38(3): 135-43. [ Links ]
10. Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd 3rd GD, Dupuy DE, Gervais D, Gillams AR, Kane RA, Lee Jr FT, Livraghi T, McGahan J, Phillips DA, RMI H, Silverman SG; Society of Interventional Radiology Technology Assessment Committee; International Working Group on Image-Guided Tumor Ablation. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology 2005; 235: 728-739. [ Links ]
11. Calkins H, Reynolds MR, Spector P, Sondhi M, Xu Y, Martin A, Williams CJ, Sledge I. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol 2009; 2(4): 349-61. [ Links ]
12. McCann CJ, Gal B, Geelen P. Ablation of a therapy-resistant posteroseptal accessory atrioventricular pathway: going for gold. Acta Cardiol 2010; 65(2): 269-70. [ Links ]
13. Cosio FG, López-Gil M, Goicolea A, Arribas F, Barroso JL. Radiofrequency ablation of the inferior vena cava-tricuspid valve isthmus in common atrial flutter Am J Cardiol 1993; 71(8): 705-9. [ Links ]
14. Lábrová R, Spinar J, Honzíková N. Radiofrequency ablation in treatment of atrial fibrillation Physiol Res 2010; 59 Suppl 1: S43-9. [ Links ]
15. Nasso G, Bonifazi R, Fiore F, Balducci G, Conte M, Lopriore V, Speziale G. Minimally invasive epicardial ablation of lone atrial fibrillation in pediatric patient. Ann Thorac Surg 2010; 90(4): e49-51. [ Links ]
16. Berjano EJ. Ablación quirúrgica. Fuentes de energía y tecnologías. Cirugía Cardiovascular 2008; 15: 375-383. [ Links ]
17. Gilson N, Honoré C, Detry O, De Roover A, Coimbra C, Kohnen L, Polus M, Piront P, Van Daele D, Honoré P, Meurisse M. Surgical management of hepatic metastases of colorectal origin. Acta Gastroenterol Belg. 2009; 72(3): 321-6. [ Links ]
18. Lyrdal D, Andersson M, Hellstrõm M, Sternal J, Lundstam S. Ultrasound-guided percutaneous radiofrequency ablation of small renal tumors: Clinical results and radiological evolution during follow-up. Acta Radiol 2010; 51(7): 808-18. [ Links ]
19. Pua BB, Thornton RH, Solomon SB. Ablation of pulmonary malignancy: current status. J Vasc Interv Radiol 2010; 21(8 Suppl): S223-32. [ Links ]
20. Gillams A. Tumour ablation: current role in the kidney, lung and bone. Cancer Imaging 2009; 9 Spec No A: S68-70. [ Links ]
21. Haemmerich D, Lee FT Jr. Multiple applicator approaches for radiofrequency and microwave ablation. Int J Hyperthermia 2005; 21(2): 93-106. [ Links ]
22. Gulesserian T, Mahnken AH, Schernthaner R, Memarsadeghi M, Weber M, Tacke A, Kettenbach J. Comparison of expandable electrodes in percutaneous radiofrequency ablation of renal cell carcinoma. Eur J Radiol 2006; 59(2): 133-9. [ Links ]
23. Burdío F, Berjano EJ, Navarro A, Burdío JM, Güemes A, Grande L, Sousa R, Subiró J, Gonzalez A, Cruz I, Castiella T, Tejero E, Lozano R, de Gregorio MA. FR tumor ablation with internally cooled electrodes and saline infusion: what is the optimal location of the saline infusion? Biomed Eng Online 2007; 6: 30. [ Links ]
24. Weber JC, Navarra G, Jiao LR, Nicholls JP, Jensen SL, Habib NA. New technique for liver resection using heat coagulative necrosis. Ann Surg 2002; 236(5): 560-3. [ Links ]
25. Burdío F, Berjano EJ, Navarro A, Burdío JM, Grande L, Gonzalez A, Cruz I, Güemes A, Sousa R, Subirá J, Castiella T, Poves I, Lequerica JL. Research and development of a new RF-assisted device for bloodless rapid transection of the liver: computational modeling and in vivo experiments. Biomed Eng Online 2009; 8: 6. [ Links ]
26. Nissen NN, Grewal N, Lee J, Nawabi A, Korman J. Completely laparoscopic nonanatomic hepatic resection using saline-cooled cautery and hydrodissection. Am Surg 2007; 73(10): 987-90. [ Links ]
27. Jiao LR, Navarra G, Weber JC, Havlik R, Nicholls JP, Habib NA. Radiofrequency assisted liver resection-a novel technique. Hepatogastroenterology 2005; 52(66): 1685-7. [ Links ]
28. Rossi P, De Majo A, Mauti A, Mauti P, Quattrini V, Mattei M, Tognoni V, Cenci L, Manzelli A, Lorenzo ND, Gaspari AL. Bloodless hepatic resection with automatic bipolar radiofrequency generator and multielectrode device. Minim Invasive Ther Allied Technol 2007; 16(1): 66-72. [ Links ]
29. Franco W, Kothare A, Goldberg DJ. Controlled volumetric heating of subcutaneous adipose tissue using a novel radiofrequency technology. Lasers Surg Med 2009; 41(10): 745-50. [ Links ]
30. Franco W, Kothare A, Ronan SJ, Grekin RC, McCalmont TH. Hyperthermic injury to adipocyte cells by selective heating of subcutaneous fat with a novel radiofrequency device: feasibility studies. Lasers Surg Med 2010; 42(5): 361-70. [ Links ]
31. Guy AW, Lehmann JF, Stonebridge JB. Therapeutic applications of electromagnetic power. Proc IEEE 1974; 62(1): 55-75. [ Links ]
32. Sadick N. Tissue tightening technologies: fact or fiction. Aesthet Surg J 2008; 28(2): 180-8. [ Links ]
33. Tillman DB, Treede RD, Meyer RA, Campbell JN. Response of c fibre nociceptors in the anaesthetized monkey to heat stimuli: Estimates of receptor depth and threshold. J Physiol 1995; 485(Pt 3): 753-765. [ Links ]
34. Plaghki L, Mouraux A. How do we selectively activate skin nociceptors with a high power infrared laser? physiology and biophysics of laser stimulation. Neurophysiol Clin 2003; 33(6): 269-277. [ Links ]
35. Hruza G, Taub AF, Collier SL, Mulholland SR. Skin rejuvenation and wrinkle reduction using a fractional radiofrequency system. J Drugs Dermatol 2009; 8(3): 259-65. [ Links ]
36. Hantash BM, Renton B, Berkowitz RL, Stridde BC, Newman J. Pilot clinical study of a novel minimally invasive bipolar microneedle radiofrequency device. Lasers Surg Med 2009; 41(2): 87-95. [ Links ]
37. Halachmi S, Orenstein A, Meneghel T, Lapidoth M. A novel fractional micro-plasma radio-frequency technology for the treatment of facial scars and rhytids: a pilot study. J Cosmet Laser Ther 2010; 12(5): 208-12. [ Links ]
38. Manstein D, Herron GS, Sink RK, Tanner H, Anderson RR. Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med 2004; 34(5): 426-38. [ Links ]
39. Berjano EJ, Navarro E, Ribera V, Gorris J, Alió JL. Radiofre-quency heating of the cornea: an engineering review of electrodes and applicators. Open Biomed Eng J 2007; 1: 71-6. [ Links ]
40. Chang JS, Lau SY. Conductive Keratoplasty to Treat Hyperopic Overcorrection After LASIK for Myopia. J Refract Surg 2010; 25: 1-7. [ Links ]
41. Ardjomand N, Wohlfart C, McAlister JC, El-Shabrawi Y, Vidic B. Conductive keratoplasty for asymmetric corneal astigmatism. J Cataract Refract Surg 2008; 34(5): 874-5. [ Links ]
42. Alió JL, Claramonte PJ, Cáliz A, Ramzy MI. Corneal modeling of keratoconus by conductive keratoplasty. J Cataract Refract Surg 2005; 31(1): 190-7. [ Links ]
43. Aziz AM, El-Khayat HR, Sadek A, Mattar SG, McNulty G, Kongkam P, Guda MF, Lehman GA. A prospective randomized trial of sham, single-dose Stretta, and double-dose Stretta for the treatment of gastroesophageal reflux disease. Surg Endosc 2010; 24(4): 818-25. [ Links ]
44. Kim DW, Yoon HM, Park JS, Kim YH, Kang SB. Radiofrequency energy delivery to the anal canal: is it a promising new approach to the treatment of fecal incontinence? Am J Surg 2009; 197(1): 14-8. [ Links ]
45. Reymunde A, Santiago N. Long-term results of radiofre-quency energy delivery for the treatment of GERD: sustained improvements in symptoms, quality of life, and drug use at 4-year follow-up. Gastrointest Endosc 2007; 65(3): 361-6. [ Links ]
46. Felt-Bersma RJ, Szojda MM, Mulder CJ. Temperature-controlled radiofrequency energy (SECCA) to the anal canal for the treatment of faecal incontinence offers moderate improvement. Eur J Gastroenterol Hepatol 2007; 19(7): 575-80. [ Links ]
47. Neruntarat C, Chantapant S. Radiofrequency surgery for the treatment of obstructive sleep apnea: short-term and long-term results. Otolaryngol Head Neck Surg 2009; 141(6): 722-6. [ Links ]
48. Back LJ, Hytõnen ML, Roine RP, Malmivaara AO. Radiofre-quency ablation treatment of soft palate for patients with snoring: a systematic review of effectiveness and adverse effects. Laryngoscope. 2009; 119(6): 1241-50. [ Links ]
49. Ceylan K, Emir H, Kizilkaya Z, Tastan E, Yavanoglu A, Uzunkulaoglu H, Samim E, Felek SA. First-choice treatment in mild to moderate obstructive sleep apnea: single-stage, multilevel, temperature-controlled radiofrequency tissue volume reduction or nasal continuous positive airway pressure. Arch Otolaryngol Head Neck Surg 2009; 135(9): 915-9. [ Links ]
50. Castro M, Musani AI, Mayse ML, Shargill NS. Bronchial thermoplasty: a novel technique in the treatment of severe asthma. Ther Adv Respir Dis 2010; 4(2): 101-16. [ Links ]
51. Miller JD, Cox G, Vincic L, Lombard CM, Loomas BE, Danek CJ. A prospective feasibility study of bronchial thermoplasty in the human airway. Chest 2005; 127(6): 1999-2006. [ Links ]
52. Castro M, Rubin AS, Laviolette M, Fiterman J, De Andrade Lima M, Shah PL, Fiss E, Olivenstein R, Thomson NC, Niven RM, Pavord ID, Simoff M, Duhamel DR, McEvoy C, Barbers R, Ten Hacken NH, Wechsler ME, Holmes M, Phillips MJ, Erzurum S, Lunn W, Israel E, Jarjour N, Kraft M, Shargill NS, Quiring J, Berry SM, Cox G; AIR2 Trial Study Group. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med 2010; 181(2): 116-24. [ Links ]
53. Sakr MF, Moussa MM. LigaSure hemorrhoidectomy versus stapled Hemorrhoidopexy: a prospective, randomized clinical trial. Dis Colon Rectum 2010; 53(8): 1161-7. [ Links ]
54. Singh P, O'Connell D, Langille M, Dziegielewski P, Allegretto M, Harris J. LigaSure versus conventional hemostasis in thyroid surgery: prospective randomized controlled trial. J Otolaryngol Head Neck Surg 2010; 39(4): 378-84. [ Links ]
55. Hartwig W, Duckheim M, Strobel O, Dovzhanskiy D, Bergmann F, Hackert T, Büchler MW, Werner J. LigaSure for pancreatic sealing during distal pancreatectomy. World J Surg 2010; 34(5): 1066-70. [ Links ]
56. Seckiner I, Yesilli C, Akduman B, Altan K, Mungan NA. A prospective randomized study for comparing bipolar plasmakinetic resection of the prostate with standard TURP. Urol Int 2006; 76(2): 139-43. [ Links ]
57. Naslund MJ, Carlson AM, Williams MJ. A cost comparison of medical management and transurethral needle ablation for treatment of benign prostatic hyperplasia during a 5-year period. J Urol 2005; 173(6): 2090-3. [ Links ]
58. Cherukuri P, Glazer ES, Curley SA. Targeted hyperthermia using metal nanoparticles. Adv Drug Deliv Rev 2010; 62(3): 339-45. [ Links ]
59. Cardinal J, Klune JR, Chory E, Jeyabalan G, Kanzius JS, Nalesnik M, Geller DA. Noninvasive radiofrequency ablation of cancer targeted by gold nanoparticles. Surgery 2008; 144(2): 125-32. [ Links ]
60. Fleming CP, Quan KJ, Rollins AM. Toward guidance of epicardial cardiac radiofrequency ablation therapy using optical coherence tomography. J Biomed Opt 2010; 15(4): 041510. [ Links ]
61. Reddy VY, Morales G, Ahmed H, Neuzil P, Dukkipati S, Kim S, Clemens J, D'Avila A. Catheter ablation of atrial fibrillation without the use of fluoroscopy. Heart Rhythm 2010; 7(11): 1644-53. [ Links ]
62. Leonelli FM, Tomassoni G, Richey M, Natale A. Usefulness of three-dimensional non-fluoroscopic mapping in the ablation of typical atrial flutter. Ital Heart J 2002; 3(6): 360-5. [ Links ]
63. Frich L. Non-invasive thermometry for monitoring hepatic radiofrequency ablation. Minim Invasive Ther Allied Technol. 2006; 15(1): 18-25. [ Links ]
64. Lyrdal D, Andersson M, Hellstrõm M, Sternal J, Lundstam S. Ultrasound-guided percutaneous radiofrequency ablation of small renal tumors: Clinical results and radiological evolution during follow-up. Acta Radiol 2010; 51(7): 808-18. [ Links ]
65. Iyer RS, Timm BA, Mitsumori LM, Kolokythas O. Image fusion as a new postprocessing method to evaluate the radio-frequency ablation zone after treatment of malignant liver tumors. J Comput Assist Tomogr 2010; 34(2): 226-8. [ Links ]
66. Mansour M, Forleo GB, Pappalardo A, Barrett C, Heist EK, Avella A, Bencardino G, Dello Russo A, Casella M, Ruskin JN, Tondo C. Combined use of cryoballoon and focal open-irrigation radiofrequency ablation for treatment of persistent atrial fibrillation: results from a pilot study. Heart Rhythm 2010; 7(4): 452-8. [ Links ]
67. Rempp H, Voigtlander M, Clasen S, Kempf S, Neugebauer A, Schraml C, Schmidt D, Claussen CD, Enderle MD, Goldberg SN, Pereira PL. Increased ablation zones using a cryo-based internally cooled bipolar RF applicator in ex vivo bovine liver. Invest Radiol 2009; 44(12): 763-8. [ Links ]
68. Hines-Peralta A, Hollander CY, Solazzo S, Horkan C, Liu ZJ, Goldberg SN. Hybrid radiofrequency and cryoablation device: preliminary results in an animal model. J Vasc Interv Radiol 2004; 15(10): 1111-20. [ Links ]
69. Andreuccetti D, Fossi R, Petrucci C. Dielectric properties of body tissues, 1997-2007. URL http://niremf.ifac.cnr.it/tissprop/. Internet database. [ Links ]
Notas
This work received financial support from the Spanish «Plan Nacional de I+D+I del Ministerio de Ciencia e Innovación», Grant No. TEC2008-01369/TEC. The authors gratefully acknowledge CONACyT (Mexico) for support through project FMSLP-C01-2007-62604 that allowed a technical visit of R. Romero-Méndez to the Universidad Politécnica de Valencia (Spain) and of E.J. Berjano to Universidad Autónoma de San Luis Potosí (México).
Este artículo también puede ser consultado en versión completa en: http://www.medigraphic.com/ingenieriabiomedica/