Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ingeniería biomédica
versión On-line ISSN 2395-9126versión impresa ISSN 0188-9532
Rev. mex. ing. bioméd vol.31 no.2 México dic. 2010
Artículo de investigación original
Single channel abdominal ECG algorithm for real-time maternal and fetal heart rate monitoring
Arias-Ortega R.*, Gaitán-González M.J.**
* Postgrado de Ing. Biomédica. Depto. de Ing. Eléctrica, UAM-Iztapalapa.
** Depto. de Ciencias de la salud. UAM-Iztapalapa.
Correspondence:
Arias Ortega R.
Av. San Rafael Atlixco, México, D.F. 09340, México
Tel: (55)58044734, Fax: (55) 58044727, E-mail: ronald.arias@gmail.com
Received article: 26/may/2010.
Accepted article: 30/november/2010.
ABSTRACT
From a single channel abdominal ECG, a maternal (MHR) and fetal heart rate (FHR) detection method is proposed. This method is based on sequential processing: detection and cancellation of maternal QRS complexes, followed by fetal QRS complexes detection, involving hardware suitable processing techniques. The algorithm was tested on a group of 25 different gestational age pregnant women signals; its performance was assessed using detections obtained by another single channel method and an expert physician. Concordance was evaluated by Cohen's kappa coefficient and sensitivity comparison was done by repeated measures ANOVA for method and gestational age (p < 0.05). Sensitivity behavior against fetal SNR was obtained as well as temporal coincidence of fetal and maternal complexes. Both methods presented similar high Cohen's kappa coefficients, with detection success rates superior to 97% and 88% for maternal and fetal complexes respectively, but for 38 weeks of pregnancy the proposed method had lower maternal success rate than reference one (90%, p > 0.05). Proposed algorithm may be implemented in an independent platform and only requires operator supervision at the beginning of the process.
Key words: Abdominal ECG, real time processing, fetal heart rate, maternal heart rate, portable device.
RESUMEN
Se propone un método para la detección de la frecuencia cardiaca fetal (FCF) y materna (FCM) partiendo de un solo canal abdominal. Consiste en una serie de pasos secuenciales: detección y cancelación de los complejos QRS maternos, seguidos de la detección de los complejos QRS fetales, involucrando técnicas de procesamiento que se pueden implementar en hardware. El método se evaluó en un conjunto de 25 registros abdominales de mujeres embarazadas con diferentes edades gestacionales. Su desempeño se comparó contra las detecciones realizadas por un experto y contra otro método de detección. El nivel de acuerdo se evaluó con el índice kappa de Cohen y la sensibilidad se comparó por análisis de varianza para muestras repetidas con métodos y edades gestacionales como factores (p < 0.05). Ambos métodos presentaron altos índices kappa, con tasas de detecciones similares, superiores al 97% y 88% para los complejos maternos y fetales respectivamente, a excepción de los registros con edades gestacionales superiores a la semana 38, donde el método propuesto presentó tasas de detecciones maternas más bajas que las de referencia (90%, p > 0.05). El algoritmo propuesto puede ser implementado en una plataforma independiente y sólo requiere de la supervisión de un operador al inicio del proceso.
Palabras clave: ECG Abdominal, procesamiento en tiempo real, frecuencia cardiaca fetal, equipo portátil.
INTRODUCTION
Maternal and fetal heart monitoring is important in order to evaluate mother and fetus health. Maternal heart rate (MHR) and fetal heart rate (FHR) are relevant variables, because through them, indicators related of the autonomic nervous system can be obtained, giving significant parameters to establish stress level in fetus1. Also, it has been determined that long-term monitor systems (e.g., 24 h), provide more information about fetal condition2. Although Doppler ultrasound is a routine technique to measure FHR during pregnancy, it is quite sensible to movements, which could reduce effectiveness in a portable system. Besides, the only parameter obtained by this technique is FHR, when it has been demonstrated that morphological and temporal parameters of fetal electrocardiogram signal (FECG), provide information about fetus health during pregnancy3.
Methods based on signal processing of abdominal electrocardiogram (AECG) have a better perspective for long term monitoring4. The main drawback for this kind of methods is the low fetal signal to noise ratio (SNR) due to interference signals. Fetal electrocardiogram (FECG) recording is disturbed by maternal electrocardiogram (MECG) that has a larger amplitude and similar spectral behavior, maternal breathing amplitude modulation, line interference, electromyography (EMG) and artifacts produced by maternal and fetal movement. Additionally, morphology of FECG depends on electrodes location, gestational age and fetus position5. Unfortunately, a standardized electrodes position to carry out the acquisition does not exist and it would be difficult to establish due to fetal movement.
To overcome these limitations, several algorithms based on processing multiple leads signals have been developed using estimation techniques like adaptive filtering6 or averaging and subtracting7. Most recent approaches applied to this problem consider FECG extraction as a blind source separation problem, so they are based on principal component analysis (PCA) and independent component analysis (ICA)8. Although these algorithms have succeeded in obtaining fetal tachogram, several leads approach could be an inconvenience for long-term monitoring9. Another approach eliminates interferences from different sources sequentially, obtaining good results when it was compared against an ICA method10.
In this paper, an alternative method is proposed based on sequential separation and detection of interest signals, considering a priori information of maternal and fetal signals. Design criteria were: use of only one abdominal lead, real-time suitable granting its implementation on microcontroller based or digital signal processor system, which must be viable as a portable system for long term monitoring. The accuracy of developed method was evaluated and compared with another single lead detection method previously created, tested and regularly used at the Human Physiology Laboratory of the Autonomous Metropolitan University.
METHODS
A. Proposed algorithm
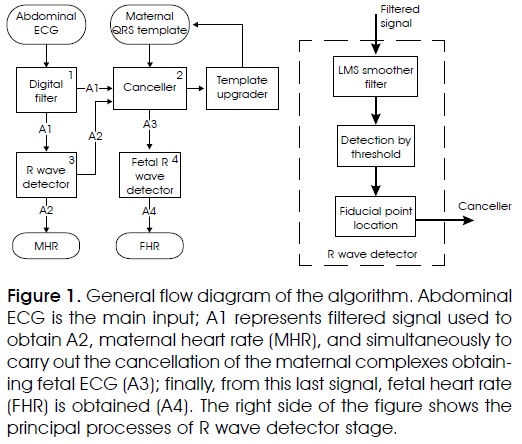
The proposed method obtains MHR and FHR by removing sequentially the interference signals involved on abdominal electrocardiogram (AECG). Figure 1 shows the block diagram with the algorithm general flow; the entire outline was implemented on Matlab®.
AECG signal, as system input, is passed through a finite impulse response (FIR) bandpass filter. The stage output (A1) represents the filtered signal used to determine maternal R wave positions (A2) through the process named QRS detector. Later on, canceller stage uses a maternal QRS template and obtained information to suppress maternal complexes on A1, getting mainly FECG signal as result (A3). This last signal is applied again to QRS detector process, adapted now to obtain fetal complexes position (A4). QRS maternal template is upgraded each beat using some predetermined parameters. The algorithm shows the first detections based on the established processes and requires an operator confirmation to continue; in case of fail in the initial detections the operator can restart the process until the algorithm gets the appropriate initial detections. Each process used in this method is presented in the following subsections.
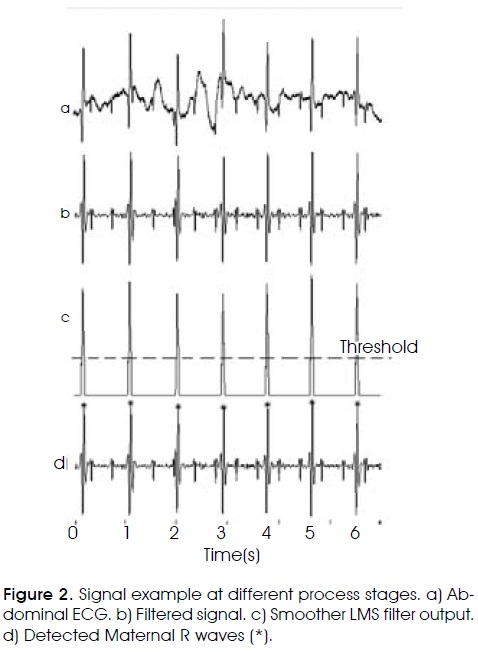
1. Preprocessing digital filter. Its objective is to remove the largest quantity of unwanted information from the AECG. Mainly, it is focused on removing baseline wander and high frequency noise. Baseline wander is caused by patient's breathing or maternal or fetal movements; its frequency range is smaller than 1 Hz. A finite response bandpass filter was designed with cut-off frequencies of 10 and 70 Hz, with order 64 using a Kaiser window; this bandwidth keeps maternal and fetal R wave information. Figure 2a shows a typical AECG signal and Figure 2b shows the filter output.
2. Maternal R wave detector. This is the main process and has three fundamental parts: (1) exploiting statistical differences between QRS complex and the rest of the ECG signal11, probable complex zone locations are highlighted; (2) these probable zones are established through a threshold; (3) maximum of QRS wave is located inside that zone. This stage is showed at the right side of Figure 1.
For the first part, a smoother least mean square (LMS) adaptive filter is used. It was designed to become unstable in regions with QRS complexes. Optimal results were obtained with a learning rate of µ = 125 x 10-6 and a filter order of 21 for 500 Hz sampling frequency. Using these parameters, the filter becomes non-stable only in the QRS region, where inverse correlation matrix maximum eigenvalue become lower than 2µ. This LMS filter receives as input U(n) the derivative of the previous filtered signal. Then, Un is a vector formed with ten previous samples U(n-10) to U(n-1) the present data U(n), and ten consecutive future data U(n+1) to U(n+10). The output of the smoother y(n), is defined as the inner product between the vector and the vector formed with the weights of the smoother Wn: y[n] = Utn Wn. At each iteration, vector Wn is updated according to the LMS algorithm, but its elements are normalized according to equation (1) to avoid that high unstable output values rise above a suitable range:
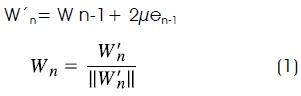
Where e[n] is the error signal e[n] = d[n] - y[n], and d[n] the desired input at time n, in this case the present signal u[n] (Figure 3). The magnitude value of the smoother output was obtained and differentiated to allow an easier detection of the QRS segments initial time11 (Figure 2c).
The second part of this stage defines a QRS searching zone. A predetermined threshold was used to settle down the R wave searching region (Figure 2c - dotted line). In the third part, when the output signal overcomes the threshold, the R wave search starts. The fiducial point is determined as the maximum absolute value from the filtered AECG signal within of searching region (Figure 2d).
In Figure 2, a signal segment where fetal complexes do not have incidence in maternal ones is shown. However, in some records fetal complexes may reach considerable amplitude and may be detected as maternal ones. Another probable problem could be the coincidence of maternal and fetal complexes that may result in the attenuation of both signals in the abdominal record. These cases make critical the searching threshold definition. Thus, the following approaches were adopted to optimize maternal complexes detection: the signal amplitude is normalized using the maximum absolute value of the two initial seconds of the filtered signal; the signal initial period should be validated by the operator so, if the algorithm has to choose among near probable detections, it would select the closest one to the current cardiac period.
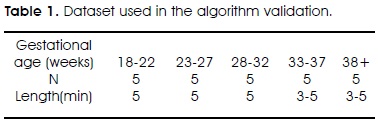
3. Canceller: This stage receives three signals: the filtered AECG (A1), current temporal maternal R wave location (A2) and maternal complex template. Maternal P, QRS and T waves have durations of 0.2s, 0.1 s and 0.40s, respectively12. In concordance, an initial template is generated; it embraces 0.1s before and after the R wave, and it was generated using several testing signals. This template is scaled according to the first maximum R wave value detected, and it is subtracted from the detected complex in the filtered AECG (Figure 4a). Template is updated each detected beat by a weighted average of current template and maternal complex just detected. To do it, standard deviation is obtained from the subtraction of the template from the maternal current complex. If standard deviation is lower than previous one, it implies an improvement in cancellation process, then template is updated using a larger current complex weight; otherwise, that weight is decreased.
4. Fetal R wave detector: It follows the same principle than maternal detector, except for the following considerations: Since cancellation procedure is stabilized after the four beat, to normalize the amplitude of input signal, the maximum amplitude one second signal around fifth maternal beat is used; starting from this point, mean time period from five detected fetal complexes that met a heart rate of 2 to 2.7 Hz (established values for fetus healthy cases7), is established as fetal heart period to use it in case of doubtful or multiple detection events, choosing the most appropriate detection according to current fetal period. The LMS filter output is smaller, so the threshold used to define the probable fetal QRS zone is lower (Figure 4b).
B. Comparison method
An algorithm already developed for maternal and fetal tachogram construction starting from AECG was used as comparison method. This algorithm requires operator attendance for visual identification of the first two maternal and fetal complexes; those serve as parameters for the maternal and fetal complexes search, applying these parameters as temporal and morphological criteria over the AECG signal13. This algorithm has an important design disadvantage: currently it presents a limitation for its implementation on an independent platform, so its use is restricted to offline processing on a computer.
C. Dataset
Two data types were used. Test signals, were obtained from PHYSIONET14, consisted on 55 records from one pregnant woman during several gestational ages (20 to 40 weeks), each record with two thoracic and three to four abdominal signals, acquired at 1000 Hz of sampling frequency. These signals were used to determine the templates and test initial algorithm performance. Validation signals came from a Human Physiology Laboratory database. It was constituted by annotated records from 65 pregnant women, 18 to 40 weeks of pregnancy, with two to four abdominal signals by subject recorded at different conditions. Records were acquired with MP100 BIOPAC module together with ECG100 electrocardiography amplifier, using a sampling frequency of 500 Hz. From this database, 25 records of different subjects were randomly chosen, obtaining five subjects for five different gestational ages, as it is shown in Table 1. Besides abdominal ECG, these records are annotated with maternal and fetal complex locations determined by an expert physician. These records allowed proposed method validation and comparison with the reference one.
D. Measures
To assess proposed algorithm performance, reference and proposed method were evaluated against detections done by an expert physician using the 25 chosen records. Each AECG signal was displayed in segments of ten to twenty seconds; the expert marked each maximum of maternal QRS complexes, first for maternal and then for fetal cases. Since fiducial point definition differs between the methods, detection was considered as correct if it remained inside 80 ms around the expert defined point for the maternal case, and 60 ms for fetal one.
Sensitivity was used as success detection rate. For this, signal was divided in segments of 100 ms for the maternal case and 80 ms for the fetal one; segments with correct detections were identified as true positives, and missed detections as false negatives. Cohen's kappa coefficient was obtained to quantify agreement level between both methods and expert detections obtaining absolute indicators for 0.95 confidence interval. This index is defined by equation (2), where Pe is the proportion of agreement expected and P0 represents the observed one15:

Repeated measures analysis of variance was used to determine differences in success rates due to methods (within factor) or gestational age (between factor) using a statistical significance 0.05.
Algorithms performance behavior was analyzed in relation to SNR measured on ECG. For maternal SNR (SNRM), MECG was considered as signal and FECG and baseline as noise16. For the fetal one (SNRF), the usual measure was used, as described below in equation (3):
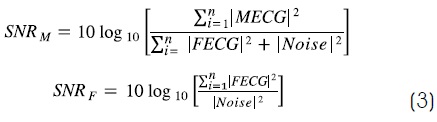
Additionally, detectors behavior was also described respect to the temporary incidence of fetal complexes inside maternal ones. Match zone was defined as 190 ms before and 75 ms after the maternal R wave17. This time interval coincided with Class C = 100% criterion proposed by Matonia18, which means overlapping is complete. The parameter could be calculated as equation (4), where (C) is the percentage of coincidence permitted (100% means any region of maternal QRS complex) and CR is the number of fetal complexes that coincide with maternal ones:

RESULTS
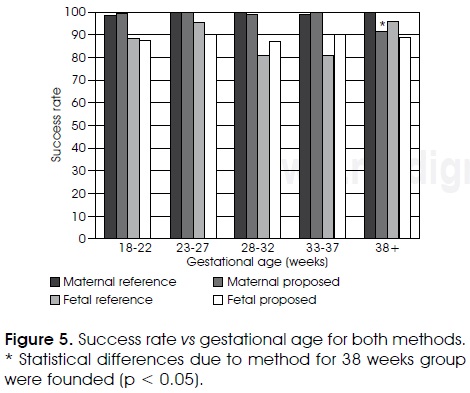
Average success detection rates for proposed and reference methods were high: 97.5% vs 99.0% for maternal case and 88.0% vs 87.2% for fetal complexes, respectively. Figure 5 shows success detection rates of both methods for the different gestational ages. Repeated measures analysis of variance showed that success rates did not present significant differences (p > 0.05) due to gestational age or method in the fetal analysis nor in the maternal case, except for the gestational ages of 38 weeks or more, where a significant difference was observed due to the method for maternal case (p < 0.05).
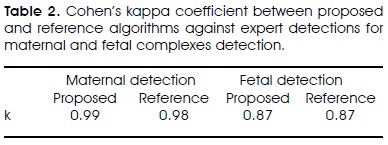
Cohen's kappa agreement coefficient of both methods with relation to expert detections corresponded to high concordance values as can be seen in Table II since high concordance is defined whenever kappa value is over 0.81. It can also be appreciated that kappa coefficients are practically the same for both methods either for maternal or fetal cases.
Figure 6 shows maternal and fetal detection performance of both algorithms vs. fetal SNR. In the same graphic, the temporal coincidence percentage of the fetal complex on maternal one and the SNR for MECG were included with descriptive purpose for discussion. High success maternal detection rates that does not depends on fetal SNR could be appreciated, while fetal sensitivity was lower and erratic for both methods. Five possible points of interest with low fetal sensitivity values were observed.
DISCUSSION
Our purpose was to develop a detection method with similar performance to reference one, but using processing techniques that allow its implementation on independent devices. As established at the results, success rates were similar for both maternal and fetal complexes detection (ANOVA test, p < 0.05), and high concordance with expert detection could be appreciated by Cohen's kappa coefficients. In regard to implementation, the most complex process in proposed algorithm, the LMS filter, that is hardware suitable, requiring similar memory and processing resources than a common FIR filter.
Proposed method bases the detection on statistic characteristics of the signal. It has certain advantages over reference method, since the latter uses signal morphology defined by the first beats of the record, so it can present poor results in high noise conditions, if the complex morphology changes, or when noise becomes similar to morphological characteristics of the signal13. On the other hand, proposed algorithm fails when fetal complexes amplitude is large, resembling maternal beats, so fetal complexes can be wrongly identified in maternal QRS detection. This may explain the lower success rate for maternal complex after 38th week, where ordinarily, records present a higher fetal signal to noise ratio; to improve this maternal detection rate additional information, such as a follow-up of both heart periods should be used.
As expected, low and high fetal SNR reduced algorithms sensitivity respect to the mean values. (Figure 6, points 1 and 5). Low fetal SNR makes detection difficult, because noise amplitude may cover fetal signal. On the other hand, high fetal SNR may be associated to large fetal complexes, which could generate false positives on the maternal QRS detection, decreasing it and consequently the correct fetal detections.
Detection behavior in points 2, 3 and 4 of Figure 6, does not have a simple interpretation. Low maternal to fetal amplitude ratio may produce false positive as was explained before, and high fetal complexes coincidence with maternal ones may reduce fetal detections due to poor maternal cancellation. A combination of these factors together with fetal SNR variations may decrease the sensitivity.
When fetal detection rates are compared with algorithms previously published, proposed algorithm presented better overall gestational weeks performance, since Martens reported detection rates of 85% and 60% for a sequential algorithm and joint approximate diagonalization of eigenmatrices algorithm respectively10, and proposed algorithm had a better rate of 88%. Besides, an important difference with those methods is that proposed algorithm is single channel while reported algorithms use five channels at least.
CONCLUSION
Proposed algorithm shows a comparable performance respect to reference method with very promising detection rates, above 97% on maternal case and 88% on fetal one.
Although both methods use single channel, a remarkable characteristic of proposed method is that it only requires the operator supervision for ensure correct determination of initial parameters, while reference method must receive initial parameters from the operator in order to execute the correct complexes detection.
Finally, it is important to emphasize the key topic in the design of this new algorithm. There is a real possibility of its migration and implementation on an independent platform. Its main and most complex process is a LMS FIR filter, which implementation can be found on innumerable digital signal processor applications. This guarantees algorithm applicability on this sort of platform. Indeed, this algorithm is been implemented in the dsPICDEM™ development board and its performance will be compared with the results presented in this paper.
REFERENCES
1. Arduini D, Rizzo G, Romanini C. Computerized analysis of fetal heart rate. Journal of Perinatal Medicine 1994; 22(suppl): 22-27. [ Links ]
2. Kosasa TS, Abou-Sayp FK, Gaylyn LM, Hale RW. Evaluation of the cost effectiveness of home monitoring uterine contractions. Obstetrics and Gynecology 1990; 76: 71-75. [ Links ]
3. Symonds EM, Sahota D, Chang A. Fetal electrocardiography. Imperial College press 2001. [ Links ]
4. Kanjilal PP, Palit S, Saha G. Fetal ECG extraction from single-channel maternal ECG using singular value decomposition. IEEE Trans Biomed Eng 1997; 44: 51-59. [ Links ]
5. Vrins F, Jutten C, Verleysen M. Sensor array and electrode selection for non-invasive fetal electrocardiogram extraction by independent component analysis. Lecture Notes in Computer Science 2004; 3195: 1017-1024. [ Links ]
6. Widrow B, Glover JR, McCool J, Kaunitz J, Williams CS, Hearn RH et al. Adaptive noise cancelling: principles and applications. Proceedings of the IEEE 1975; 63: 1692-1716. [ Links ]
7. Abboud S, Beker A. An improved detection algorithm in fetal electrocardiography. J Electrocardiol 1989; 22(Suppl.): 23-842. [ Links ]
8. Zarzoso V, Nandi AK. Noninvasive fetal electrocardiogram extraction: Blind separation versus adaptive noise cancellation. IEEE Transactions on Biomedical Engineering 2001; 48: 12-18. [ Links ]
9. Ibrahimy MI, Firoz A, Mohd Ali MA, Edmond Z. Real-time signal processing for fetal heart rate monitoring. IEEE transactions on biomedical engineering 2003; 50: 258-62. [ Links ]
10. Martens SMM, Rabotti C, Mischi M, Sluijter RJ. A robust fetal ECG detection method for abdominal recordings. Physiological Measurement 2007; 28: 373-388. [ Links ]
11. Gaitán MJ, Carrasco S, González CR, Yañez SO. Detección de onda R por diferenciación estadística del complejo QRS. IV Simposio del Departamento de Ciencias de la Salud Univ. Autónoma Metropolitana Iztapalapa 1998. [ Links ]
12. Guyton AC, Hall JE. Textbook of Medical Physiology. Philadelphia, PA: Saunders 1996. [ Links ]
13. Rodríguez R. Algoritmo para la construcción en tiempo real del cardiotacograma materno y fetal a partir del ECG abdominal. Master's thesis, Universidad Autónoma Metropolitana Iztapalapa 1997. [ Links ]
14. Goldberger AL, Amaral LAN, Glass L, Hausdorff JM, Ivanov PCh, Mark RG et al. Physiobank, physiotoolkit, and physionet: Components of a new research resource for complex physiologic signals. Available: http://circ.ahajournals.org/cgi content/full/101/23/e215. [ Links ]
15. CohenJ. A coefficient of agreement for nominal scales. Educ Psychol Meas 1960; 20: 37-36. [ Links ]
16. Sargolzaei S, Faez K, Sargolzaei A. Signal processing based techniques for fetal electrocardiogram extraction, BioMedical Engineering and Informatics: New Development and the Future - Proceedings of the 1st International Conference on BioMedical Engineering and Informatics, BMEI 2008; 2: 492496. [ Links ]
17. Solum T, Ingermarsson I, Nygren A. The accuracy of abdominal ECG for fetal electronic monitoring. Journal of Perinatal Medicine 1980; 8(3): 142-149. [ Links ]
18. Matonia A, Kupka T, Jezewski J, Horoba K, Wrobel J, Gacek A. The influence of coincidence of fetal and maternal QRS complexes on fetal heart rate reliability. Medical and Biological Engineering and Computing 2006; 44(5): 393-403. [ Links ]
Nota
Este artículo también puede ser consultado en versión completa en: http://www.medigraphic.com/ingenieriabiomedica/