INTRODUCTION
Sea slugs are a group of gastropod mollusks that are classified in the subclass Heterobranchia (Class: Gastropoda). Sea slugs are characterized by presenting a reduced shell and ectodermal surface covered, or not, with parapodia. In this group of gastropods, the body morphology, size, and color are highly diverse (Valdés et al., 2006). They are common inhabitants of coral reefs, benthic algae, drifting sargassum, seagrass, rocks, and dead coral. Gastropods feed mainly on invertebrates (including other gastropods), small fish, and algae (Valdes et al., 2006; Sanvicente-Añorve et al., 2012a; Zamora-Silva & Ortigosa, 2012). Currently, there is much interest in the study of sea slugs, since they are important components of marine biodiversity (Crocetta et al., 2015). Ecologically, sea slugs carry out specific biological interactions since many species are algae eaters and some others are capable of keeping chloroplasts in a simbiotyc relationship named kleptoplastidy (Krug et al., 2016). Also, many species of sea slugs have been found to possess secondary metabolites with potential use in biomedicine (Avila & Angulo-Preckler, 2020).
Anatomical and molecular studies have also contributed to the description and identification of new species/species complexes (Golestani et al., 2019). For the reefs of the southwest of the Gulf of Mexico, most studies on sea slugs are focused on taxonomic lists. For the Bank of Campeche and Alacranes reef, there is a greater knowledge of the richness of species (Sanvicente-Añorve et al., 2012b; Ortigosa et al., 2013; 2015) with a current record of 94 species of sea slugs (Ortigosa & Simões, 2019). Two of three reef systems of Veracruz concentrate more investigations about sea slugs, being the Veracruz reef system (VRS) the best studied (Zamora-Silva & Ortigosa, 2012; Aguilar-Estrada et al., 2014; Cruz-López et al. 2015; Vital-Arriaga, 2016; Barrera-Correa, 2018; Olmos-García et al., 2019). For the Lobos-Tuxpan reef system, located at the north end of Veracruz state, there are few investigations that provide data about sea slugs species.
The first records of sea slugs for the Lobos-Tuxpan Reef System Protected Natural Area (LTRS) appeared in a general list of mollusks (Tunnell, 1974; De la Cruz-Francisco & González-Gándara, 2006; Tunnell et al., 2007) as well as in two works of macrobenthic communities of the Lobos reef (Chávez et al., 1970; De la Cruz-Francisco, 2013). Besides these investigations, actualized records are lacking for the Lobos reef. On the other hand, for the Tuxpan and Oro Verde reefs there are recent reports that provide the first record of 11 species of sea slugs (De la Cruz-Francisco et al., 2017b). It is worth mentioning that near LTRS, many species of sea slugs have been reported for the Tampamachoco lagoon (De la Cruz-Francisco et al., 2020a) and for the rocky coast of Cazones, Veracruz (De la Cruz-Francisco et al., 2017a). Although the current information on sea slugs for LTRS is valuable, it is still necessary to unify it and update it with new unpublished data. An updated list will contribute to the knowledge of marine species and ecosystems as well as to the implementation of effective conservation and protection actions (Crocetta et al., 2015). Therefore, the purpose of this work is to provide an updated list of sea slug species for the LTRS.
MATERIALS AND METHODS
The Lobos-Tuxpan reef system (LTRS) is located at the north end of Veracruz state, Mexico, off the coasts of the municipalities of Tuxpan and Tamiahua. It is part of the Reef Corridor of the Southwest Gulf of Mexico (RCSGM), which integrates another two reef complexes distributed on the central and southern coasts of Veracruz: the Veracruz (VRS) and Los Tuxtlas (TRS) reef systems (Ortiz-Lozano et al., 2013, 2019). The LTRS is a natural protected area created by a presidential decree as an Area of Protection of Flora and Fauna, and is divided into: a) the Tuxpan reef polygon, which includes the Tuxpan, Enmedio, Pantepec and Tanhuijo reefs; and b) the Lobos reef polygon, comprising the Lobos, Enmedio and Blanquilla reefs (Fig. 1). Adjacent to the polygon of protection there are three submerged reefs: Oro Verde, Pantepec Sur, and Corazones, which have recently been mapped (Ortiz-Lozano et al., 2019).
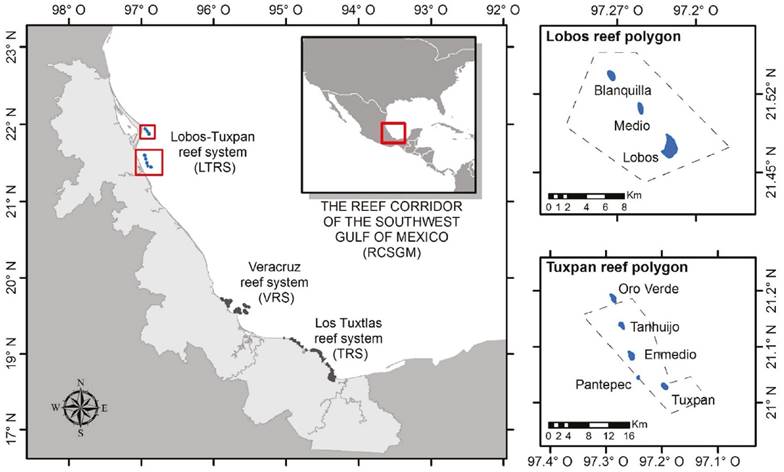
Figure 1 Geographic location of the Lobos-Tuxpan Reef System (LTRS) in the Reef Corridor of the Southeastern Gulf of Mexico.
Sea slugs were sought in the three emerged reefs of the LTRS. The greatest sampling effort was conducted in the Lobos reef, with 30 accumulated hours of field work (during November 2014 and 2017), followed by the Enmedio reef with 23 h (September 2016, April 2017, June 2018, July and November 2019) and the Tuxpan reef with 14 h (June and October 2016, and April 2017). The direct method was mainly applied, which consisted of direct observation of the species that live on sand, coral rock, and in areas of massive corals at depths of 1-3 m (Goodheart et al., 2016). To search for small species with cryptic coloration (camouflage), the indirect method is used, which consists of checking rocks, rubble, sea grass and macroalgae (Camacho-García et al., 2014; Ortigosa et al., 2015). However, neither macroalgae nor seagrasses were collected in this study.
Given that the LTRS is a natural protected area, no organisms were collected. Thus, most of the specimens observed were identified in the field through previous training based on patterns of coloration and of external morphological structures such as cerata, gill position, rhinophores, oral tentacles and surface of the notum, following the existing literature for the Gulf of Mexico and Caribbean (Carmona et al., 2011; Zamora-Silva & Ortigosa, 2012; Camacho-García et al., 2014; Caballer-Gutiérrez et al., 2015; Ortigosa et al., 2015; Goodheart et al., 2016). Likewise, specialized literature was used to distinguish complexes of species (Ornelas-Gatdula et al., 2012; Goodheart et al., 2015; Krug et al., 2016; 2018; Valdés et al., 2017; Zamora-Silva & Malaquias 2017; Golestani et al., 2019; Bazzicalupo et al., 2020). Additionally, photographic evidence of the species observed in their natural habitat was obtained using a Canon PowerShot D30 camera. The photographic material obtained was a useful tool to confirm the identity of the species based on the coloration and external morphology (Carmona et al., 2011; Ortigosa et al., 2013; Camacho-García et al., 2014).
With the information obtained from the samplings, a systematic list of the identified species was elaborated and it was complemented with the records provided by Tunnell (1974), Tunnell et al. (2007), De la Cruz-Francisco & González-Gándara (2006), De la Cruz-Francisco (2013), De la Cruz-Francisco et al. (2017a), for the purpose of compiling all species records and updating the taxonomic list of sea slugs for the LTRS. The systematic arrangement at the order level follows the criteria of Bouchet et al. (2017), while the species within each order were listed alphabetically. The nomenclature of the scientific names and synonyms was verified with the World Register of Marine Species (WoRMS Editorial Board, 2021). Species that were new records for the LTRS y RCSGM are described including the following data: systematics, material examined, sampling date, habitat, description of morphology, geographic distribution, and observations.
RESULTS
In the present study, a total of 17 species of sea slugs were identified that correspond to the Tuxpan, Enmedio and Lobos reefs. Complementing these results with the bibliographic compilation, the updated list of sea slugs now is composed of 27 species (see Table 1). At the reef level and in descending order with the species richness, 13 records are added for the Lobos reef; this raises the richness to 23 species of sea slugs. For the Tuxpan reef it increased to 13 species with the addition of nine more records, while for the Enmedio reef it increased to 11 species with the addition of four more sea slug records (Table 1).
Table 1 Systematic list of sea slugs from the Lobos-Tuxpan reef system. Records of this study (●). Bibliographic sources: A) Tunnell (1974); B) Tunnell et al. (2007); C) De la Cruz-Francisco and González-Gándara (2006); D) Zamora-Silva and Ortigosa (2012); E) De la Cruz-Francisco (2013); F) Aguilar-Estrada et al. (2014); G) Cruz-López et al. (2015); H) Vital-Arriaga (2016); I) De la Cruz-Francisco et al. (2017a); J) Barrera-Correa (2018); K) Olmos-García et al. (2019).
| Species | Tx | Em | Tj | OV | L | Previous records in the RCSGM |
|---|---|---|---|---|---|---|
| Order Pleurobranchida | ||||||
| 1. Pleurobranchus areolatus Mörch, 1863 | ● | ●, A, B, E | G, H, K | |||
| Order Nudibranchia | ||||||
| 2. Phyllidiidae sp. | I | |||||
| 3. Flabellina engeli Ev. Marcu & Er. Marcus, 1968 | ● | I | ● | H | ||
| 4. Tyrinna evelinae (Er. Marcus, 1958) | I | |||||
| Order Cephalaspidea | ||||||
| 5. Acteocina candei (d’Orbigny, 1841) | C | D, G | ||||
| 6. Atys riiseanus Mörch, 1875 | C | G | ||||
| 7. Bulla occidentalis A. Adams, 1850 | ● | ● | C | D, F, G, H, K | ||
| 8. Chelidonura hirundinina (Quoy & Gaimard, 1833) | I | ● | ||||
| 9. Haminoea antillarum (d’Orbigny, 1841) | C | D, G | ||||
| 10. Haminoea elegans (Gray, 1825) | ● | C | D, G | |||
| 11. Haminoea succinea (Conrad, 1846) | C | D, G | ||||
| 12. Navanax gemmatus (Mörch, 1863) | I | I | I | I | ●, E [as Navanax aenigmaticus (Bergh, 1893)] | D, G, H, K |
| 13. Pyrunculus caelatus (Bush, 1885) | C | |||||
| Order Aplysiida | ||||||
| 14. Aplysia cf cervina (Dall & Simpson, 1901) | A, B | G | ||||
| 15. Aplysia dactylomela Rang, 1828 | I | I | I | ●, A, B, C | D, F, G, H, K | |
| 16. Aplysia fasciata (Verrill, 1901) | ● | ●, A, B, C | G, H, K | |||
| 17. Aplysia ghanimiiGolestani, Crocetta, Padula, Camacho, Langeneck, Poursanidis, Pola, Yokeş, Cervera, Jung, Gosliner, Araya, Hooker, Schrödl & Valdés, 2019 | ● | I | C [as Aplysia parvula Mörch, 1863] | |||
| 18. Aplysia morio (Verrill, 1901) | I | K | ||||
| 19. Bursatella leachii Blainville, 1817 | ● | ●, A, B, C, [as Bursatella leachii plei (Rang, 1828)] | D, G, K | |||
| 20. Dolabrifera cf. ascifera (Rang, 1828) | ● | ●, A, B [as Dolabrifera dolabrifera (Rang, 1828)] | D, G, H, K | |||
| Superorder Sacoglossa | ||||||
| 21. Cyerce habanensisOrtea & Templado, 1988 | ● | ● | J | |||
| 22. Elysia cornigera Nuttall, 1989 | ● | H | ||||
| 23. Elysia crispata (Mörch, 1863) | I | I | I | I | ●, A, B, E | D, F, G, H, J, K |
| 24. Elysia subornata Verrill, 1901 | C | D, G, K | ||||
| 25. Lobiger souverbii P. Fischer, 1857 | ● | ● | NR | |||
| 26. Oxynoe antillarum Mörch, 1863 | I | I | ● | D, G, H | ||
| 27. Thuridilla picta (Verrill, 1901) | ● | I | ● | |||
| Total number of species | 13 | 11 | 5 | 4 | 23 |
Abbreviations: Tuxpan (Tx), Enmedio (Em), Tanhuijo (Tj), Oro Verde (OV), Lobos (L) reefs, new record (NR).
The sea slugs Cyerce habanensisOrtea & Templado, 1988 and Elysia cornigera Nuttall, 1989 constitute the first records for the protected natural area. In addition, a new record for the biological corridor is added: Lobiger souverbii P. Fischer, 1857 (Table 1). The classification, morphology, and geographic distribution of these species are described later in this section. Most of the species of sea slugs observed in the present study are shown in Figure 2.

Figure 2 Sea slugs from the Lobos-Tuxpan reef system observed in the present study; A. Pleurobranchus areolatus; B. Flabellina engeli; C. Chelinodura hirundinina; D. Navanax gemmatus; E. Aplysia dactylomela; F. Aplysia fasciata; G. Aplysia ghanimii; H. Bursatella leachii; I. Dolabrifera cf. ascifera; J. Elysia crispata; K. Thuridilla picta; L. Oxynoe antillarum.
Several species were found under coral rocks on the reef plain, such as Pleurobranchus areolatus Mörch, 1863 (Fig. 2a), Flabellina engeli Ev. Marcus & Er. Marcus, 1968 (Fig. 2b), Dolabrifera cf. ascifera (Rang, 1828) (Fig. 2i), Thuridilla picta (A. E. Verrill, 1901) (Fig. 2k), C. habanensis (Fig. 3) and E. cornigera (Fig. 4). Those that were frequently observed on the rocky and sandy substrates were Navanax gemmatus (Mörch, 1863) (Fig. 2d), Aplysia dactylomela Rang, 1828 (Fig. 2e), Aplysia fasciata Poiret, 1789 (Fig. 2f) and Aplysia ghanimiiGolestani, Crocetta, Padula, Camacho, Langeneck, Poursanidis, Pola, Yokeş, Cervera, Jung, Gosliner, Araya, Hooker, Schrödl & Valdés, 2019 (Fig. 2g). Meanwhile, Bursatella leachii Blainville, 1817 (Fig. 2h) was observed on muddy and sandy sites. On the contrary, Elysia crispata Mörch, 1863 (Fig. 2j) was one of the species observed in rocky and sandy substrates and in seagrass beds. Regarding the species that were observed in the macrophytobenthos were Chelinodura hirundinina (Quoy & Gaimard, 1833) (Fig. 2c), Haminoea antillarum (d’Orbigny, 1841), Bulla occidentalis A. Adams, 1850 in Thalassia testudinum K.D.Koening meadows, while Oxynoe antillarum Mörch, 1863 (Fig. 2l) and Lobiger souverbii P. Fischer, 1857 (Fig. 5), were observed specifically in fronds of Caulerpa spp.
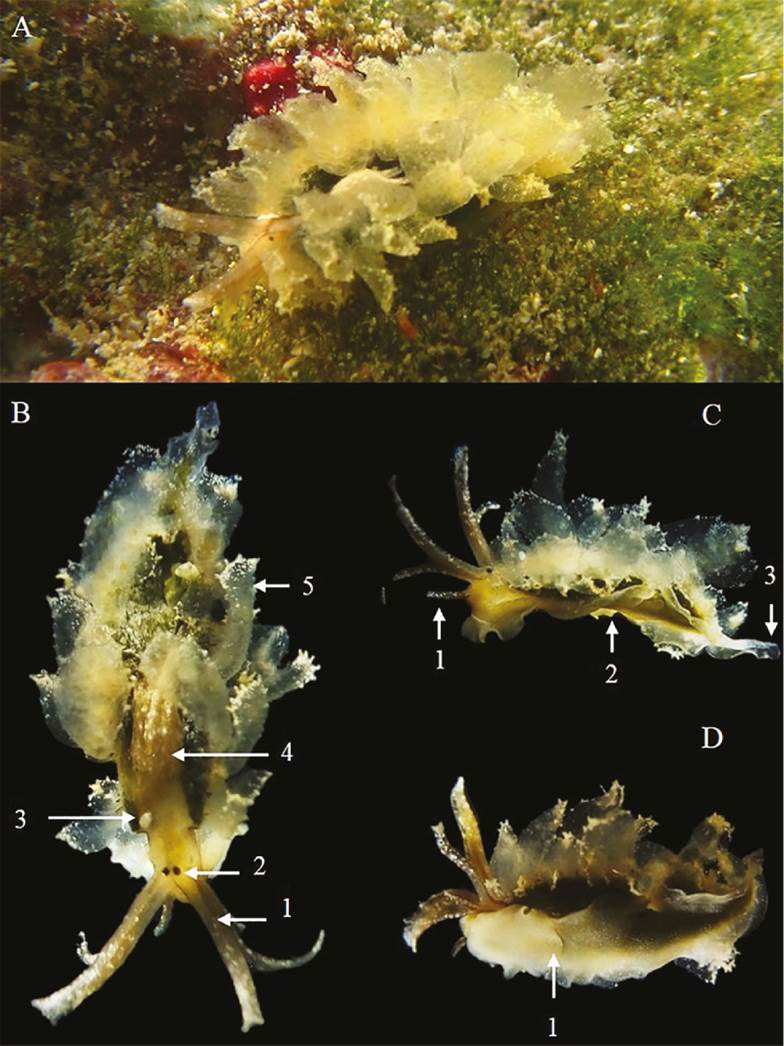
Figure 3 A. Coloration of Cyerce habanensis; B. Tuxpan reef specimen, 1: rhinophores, 2: eyes, 3: anal papila, 4: pericardium, 5: cerata. C. Lateral view, 1: bucal palp, 2: muscular foot, 3: tail. D. View of the muscular foot, 1: muscular foot is divided by a transverse groove.
Systematics
Superorder: Sacoglossa Ihering, 1876
Superfamily: Plakobranchoidea Gray, 1840
Family: Caliphyllidae Tiberi, 1881
Genus: Cyerce Bergh, 1870
Species: Cyerce habanensisOrtea & Templado, 1988
Material examined. Three individuals observed in the lagoon of Lobos reef 21°27’ 58” North, 97°13’ 35.64” West), in November 2017. One specimen was observed in Tuxpan reef (21° 1’ 40.20” North, 97° 11’ 49.69” West), September 2019.
Habitat. Found at 0.5-1 m depth on fronds of Halimeda opuntia (Linnaeus) J. V. Lamouroux and below rocks.
Morphological description. Oval and elongated body, dark green dorsal surface with long transparent cerata (Fig. 3a). Size of 11 mm in length and 5 mm in width. Rhinophores elongated and bifurcated in the middle, with white and granular papillae (Fig. 3-b1). Eyes located at the base of the rhinophores (Fig. 3-b2). The head is small and presents two long buccal palps (Fig. 3-c1). Anal papilla in anterodorsal position, located on the right side, just in front of the pericardium (Fig. 3-b3). Prominent anterodorsal pericardium, raised above the rest of back, covered with small white papillae (Fig. 3-b4). Nine cerata are present; these are flat, foliaceous, transparent and of unequal sizes, located on the lateral edges of the body. In each cerata, reddish marks are presented at the base and in the upper middle part, and the distal edge has white granules (Fig. 3-b5). Muscular foot of yellow color (Fig. 3-c2), the anterior end is rounded and divided by a transverse groove (Fig. 3-e1), the posterior end is short and triangular (Fig. 3-c3).
Geographic distribution. Cuba (Ortea & Templado, 1988; Espinosa, 2012). Mexico: Santiaguillo reef reported as Cyerce antillensis Engel, 1927 (Barrera-Correa, 2018). New record for the LTRS.
Comments. Cyerce habanensis was originally described by Ortea and Templado (1988), but Valdés et al. (2006) consider it as a synonym of C. antillensis. However, recent scientific investigations (Espinosa, 2012; Caballer et al., 2015) proposed C. habanensis as a valid name and point out that there is not taxonomic discussion that supports C. habanensis being synonymous with C. antillensis. Moreno (2020) carried out a morphological and molecular study with 154 Cyerce specimens, and his results reveal that C. antillensis and C. habanensis are different and sympatric species in the Caribbean. Therefore, we consider that the specimens observed in this study correspond to C. habanensis since they correspond with the original description by Ortea & Templado (1988) and with the morphological characteristics described by Moreno (2020). Both species can be distinguished externally, C. habanensis presents broad and translucent yellow cerata, while C. antillensis presents translucent and elongated white cerata (Ortea & Templado, 1988; Moreno, 2020). This species is morphologically similar to the substrate, also, the transparency and small size of those species makes it difficult to observe it among the fronds of macroalgae and on coralline rocks (Fig. 3a). In this study, C. antillensis was found under coralline rocks and later in H. opuntia fronds. It is often found on H. opuntia fronds, suggesting that it probably feeds on this calcareous macroalgae (Ortea & Templado, 1988).
Family: Plakobranchidae Gray, 1840
Genus: Elysia Risso, 1818
Species: Elysia cornigera Nuttall, 1989
Examined material. One specimen observed and photographed (Fig. 4) on the flat of the Enmedio reef (21° 4’ 47.84” North, 97°15’ 17.24” West), in October 2014.

Figure 4 A. Coloration and morphology of Elysia cornigera, 1: rhinophores, 2: parapodia, 3: papillae, 4: pericardium. B. Light and dark red markings on the parapodia.
Habitat. Under coralline rock, at 1 m depth.
Morphological description. Body of yellowish gray colors, narrowed and elongated, of 10 mm in length, the surface is granular with light and dark red marks, which extend over the rhinophores, head and parapodia (Fig. 4-a, b). The rhinophores are long and have a sharp apex; the surface with scattered papillae and red spots. (Fig. 4-a1). Parapodia wide, covering the dorsal position of the body, with large, sharp, white papillae with numerous scattered large red marks (Fig. 4-a2, a3). Pericardium white, rounded, in anterodorsal position, with small papillae and red marks (Fig. 4-a4).
Geographic distribution. Florida, Bahamas, Jamaica (Clark, 1994; Krug et al., 2011; 2016), Mexico: Alacranes reef (Rosenberg et al., 2009; Sanvicente-Añorve et al., 2012b; Ortigosa & Simões, 2019), Gallega reef (Vital-Arriaga, 2016). New record for the LTRS.
Comments. The morphological characteristics described in this study matches with those described by Krug et al. (2011; 2016). In several scientific investigations, E. cornigera was confused with Elysia timida (Risso, 1818), because Ortea et al. (1997) synonymized E. cornigera with E. timida. Subsequently, molecular studies determined that E. timida and E. cornigera are different species (Krug et al., 2011; 2016). Externally, E. cornigera resembles E. timida, except for the size color. The latter is much larger (6-13 mm), with a white body and a green notum; their rhinophores and parapodia are smooth with small red markings, and the tips of the rhinophores are broad and rounded. E. timida is distributed in the Mediterranean Sea (Krug et al., 2011). In contrast, E. cornigera has scattered red marks and numerous papillae on the parapodia, head and rhinophores, while the body is grey. These characteristics coincide with the descriptions of Krug et al. (2011; 2016). The habitat of this species is associated with small chlorophyte algae of Acetabularia sp., normally with Acetabularia crenulata J.V.Lamouroux (Krug et al., 2011). However, in this study it was observed under coralline rocks.
Superfamily: Oxynooidea Stoliczka, 1868
Family: Oxynoidae Stoliczka, 1868
Genus: Lobiger Krohn, 1847
Species: Lobiger souverbii P. Fischer, 1857
Material examined. Two specimens observed and photographed (Fig. 5a-b) on the flat of Lobos reef (21° 27’ 59.07” North, 97° 13’ 39.15” West), in November 2014.

Figure 5 Coloration and morphology of Lobiger souverbii (A-B), 1: shell, 2: rhinophores, 3: parapodia, 4: papillae, 5: tail (extension of the muscular foot).
Habitat. On fronds of the macroalga Caulerpa racemosa (Forsskål) J. Agardh, 1873 at 0.5 m depth.
Morphological description. Green body, presenting a small shell with dark longitudinal irregular lines (Fig. 5-b1). Long rhinophores covered with small white papillae (Fig. 5-b2). Two pairs of long, erect, protruding parapodia with wavy edges covered with white papillae on the margins (Fig. 5-b3, b4). Muscular foot that extends backwards and forms a long thick tail, also with white papillae (Fig. 5-b5).
Geographic distribution. Brazil, Venezuela, Costa Rica, Panama, Florida, Bahamas, Cuba, Jamaica (Clark, 1994; Valdés et al., 2006; Camacho-García et al., 2014; Caballer-Gutiérrez et al., 2015; Goodheart et al., 2016; de Sisto et al., 2016), Mexico: Yucatán (Rosenberg et al., 2009; Ortigosa et al., 2013). New record for the RCSGM.
Comments. The morphological characteristics described in this study agrees with those observed by Goodheart et al. (2016) and de Sisto et al. (2016). Externally, L. souverbii resemble Oxynoe spp. as they have an elongated green body and have a shell; they also inhabit and feed on the fronds of Caulerpa (Krug et al. 2018). However, L. souverbii differs from Oxynoe spp. by having two pairs of long, upright parapodia (Jensen, 2011; de Sisto et al., 2016). The external morphology of L. souverbii resembles the fronds of C. racemosa in which it is commonly found (Caballer-Gutiérrez et al., 2015, Goodheart et al., 2016). It can be distinguished from fronds when it extends its parapodia (Goodheart et al., 2016). This species has also been reported on Caulerpa sertularioides (S.G. Gmelin) M.Howe (de Sisto et al., 2016) and Halimeda sp. (Marcus & Hughes, 1974).
DISCUSSION
With our study, the number of LTRS sea slugs increased, from 24 previously reported species (Tunnell, 1974; Tunnell et al., 2007; De la Cruz-Francisco, 2013; De la Cruz-Francisco et al., 2017b), the richness has increased to 27 species (considering the Phyllidiidae family). However, the richness is lower compared with the 40 species reported for the VRS (Vital-Arriaga, 2016; Barrera-Correa, 2018; Olmos-García et al., 2019). Between these two reef systems, at least 20 species reported in our study are present in the VRS.
The sea slug richness in the LTRS is of Caribbean influence, since all of the species are found commonly in this geographic province (Valdés et al., 2006; Caballer-Gutiérrez et al., 2015; Goodheart et al., 2016; Caviedes et al., 2019; Ortigosa & Simões, 2019). While the new records (C. habanensis, E. cornigera and L. souverbii) are widely distributed in the Caribbean, and have recently been reported in the reefs of the Campeche Bank (Sanvicente-Añorve et al., 2012b, Ortigosa et al., 2013, Ortigosa & Simões, 2019), the present study widens the distribution of these species towards a more septentrional portion of the southern Gulf of Mexico.
Moreover, the superorder Sacoglossa is more diverse in the LTRS, with seven species recorded in this study versus five species recorded for the VRS (Zamora-Silva & Ortigosa, 2012; Aguilar-Estrada et al., 2014; Cruz-López et al., 2015; Vital-Arriaga, 2016; Barrera-Correa, 2018; Olmos-García et al., 2019). Only Elysia chlorotica, Gould, 1870 (the most representative “solar-powered sea slug”) and Elysia ornata (Swainson, 1840) are not present in the LTSR. These two species are not common in RCSGM and have only been reported on a single occasion for the VRS (Aguilar-Estrada et al., 2014; Cruz-López et al., 2015). They are small slugs with a cryptic coloration that makes them very difficult to observe, therefore, specialized indirect methods are required to find them and update their distribution (Ortigosa et al., 2015). Most Sacoglossa species reported for the southwestern Gulf of Mexico are herbivores and associated with several species of algae such as Rhipocephalus, Penicillus, Avrainvillea, Udotea, Gracilaria, and Padina (Sanvicente-Añorve et al., 2012a; Zamora-Silva & Ortigosa, 2012; Ortigosa et al., 2015). Therefore, to improve the sampling effort, it is important to broaden the search, specifically in these algae that are also distributed in the LTSR (De la Cruz-Francisco et al., 2020b).
Although the Cephalaspidea order is the most diverse in species in the LTRS, only three species were observed in this study, Bulla occidentalis A. Adams, 1850, C. hirundinina and N. gemmatus, the latter being the most commonly found in the reef system (Zamora-Silva & Ortigosa, 2012; De la Cruz-Francisco et al., 2017b). The rest of the species reported for the reefs of Veracruz (De la Cruz-Francisco & González-Gándara, 2006; Zamora-Silva & Ortigosa, 2012; Cruz-López et al., 2015) were not found in this study. This can be attributed to the characteristics of the benthic substrate in which they live, generally, Cephalaspidea gastropod slugs (Atys spp., Bulla spp., Haminoea spp.) live on sandy muddy bottoms and seagrass meadows (García-Cubas & Reguero, 2004), therefore, it is required to collecting sediment samples to record them, but this indirect method was not used in this study. For that reason, it is recommended that future studies intensify the search for heterobranchia slugs by applying the indirect method examining the sediments, incluiding rubble, rhizomes and fronds of macroalgae (e.g. Lobophora sp., Laurencia sp., Penicillus spp., Caulerpa sp., Halimeda spp., Acetabularia spp., etc.), as well as sponges and hydroids (Sanvicente-Añorve et al., 2012a).
All six Aplysiida species recorded in the LTRS are also present in the VRS, (Zamora-Silva & Ortigosa, 2012; Aguilar-Estrada et al., 2014; Cruz-López et al., 2015; Olmos-García et al., 2019). These sea hares have several peculiarities that contrasts with the substrate, such as their large size and coloration (green, brown or bronze), which makes it easy to find them on reefs. They also inhabit various substrates such as sea grasses and macroalgae where they are commonly observed, with A. dactylomela and B. leachii being the most frequent in these places (Sanvicente-Añorve et al., 2012a; Zamora-Silva & Ortigosa 2012; De la Cruz-Francisco et al., 2017b). However, in this study, samples of sea grasses and macroalgae were not collected, substrates in which four species were found in the SAV (Zamora-Silva & Ortigosa, 2012) and which are not present in LTRS. Therefore, it is suggested to broaden the search for sea hares with specialized indirect methods, especially with the collection of different benthic substrates in order to find more unknown species.
In relation to the order Nudibranchia, in this study three species are reported for the LTRS, of which only F. engeli is among the seven species of nudibranchs reported for VRS (Zamora-Silva & Ortigosa, 2012; Cruz-López et al., 2015; Vital-Arriaga, 2016). The low richness of nudibranchs recorded in the study area can be attributed to the fact that they were found only with the direct method. Most nudibranchs are small and cryptic (Camacho et al., 2014; Goodheart et al., 2016), feed on sponges, cnidarians (hydrozoans, anemones, and gorgonians), bryozarians and ascidians (Nybakken & McDonald, 1981; Belmonte et al., 2015), however, these sessile organisms were not collected for review, so it is important to broaden the search for nudibranchs in these sessile invertebrates in further studies.
This study provides new records of heterobranchia for the LTRS; however the list of species could be enriched with better sampling effort. A similar situation occurs with VRS, since the richness has increased to 40 species with the addition of recent records (Vital-Arriaga, 2016; Barrera-Correa, 2018; Olmos-García et al., 2019). This richness may continue to increase with more studies, as suggested by the work of Zamora-Silva & Ortigosa (2012) who propose the existence of at least 60 species of heterobranchs in this reef system. We also suggest the existence of more sea slugs in the LTRS, therefore, the continuity of the sampling projects is essential, also considering that there are other adjacent unexplored sites such as the Pantepec, Corazones, Blake and Piedras Altas reefs (Ortiz-Lozano et al., 2019). It is worth mentioning that in protected natural areas it is essential to have updated and complete inventories of the marine biota to assess changes in biological communities. They also constitute tools that support biological monitoring in management and protection decision making (Crocetta et al., 2015).











 nova página do texto(beta)
nova página do texto(beta)


