INTRODUCTION
Nutrient concentrations play a fundamental role in the growth of any phytoplankton population. So, the availability of nutrients is of great importance in determining the competitive capacity of algae (Baldia et al., 2003). Algal blooms and their causes are different in temperate zones, where they occur in the warmer months and persist throughout the summer, in contrast to those occurring in tropical zones, where they can occur at any time of the year and persist for a few weeks (Mowe et al., 2015). Excess nutrients, through anthropogenic inputs, are known to be a fundamental factor in the prevalence of these growths (Reynolds, 1984; Millie et al., 1999). Often these growths, which are generally associated with cyanobacteria such as Microcystis, Anabaena, and Cylindrospermosis, are detrimental (Mowe et al., 2015). However, there are other uncommon blooms of euglenoids, diatoms, and Chlamydomonas; some authors classify the latter genus as forming “Harmful Algal Blooms” (HABs), as they frequently clog water purification filters (Kruskopf & Du Plessis, 2004). Species of the genus Chlamydomonas, unlike cyanobacteria, are not toxic in the strict sense, but they do produce certain allelopathic compounds that inhibit the growth of other microalgae and some zooplankton organisms (Barreiro & Hairston Jr. 2013). There are few records of blooms generated by Chlamydomonas, but proliferations have occurred mainly in Europe and Australia; for example, in backwaters of the Ruhr River in Germany (Herrman & Jüttner, 1977); in lagoons and canals in the Llobregat delta, Spain, in 1982 (Catalán, 1984); in the Vaal River, South Africa, in August 1992 (Pieterse & Janse van Vuuren, 1997); and in the Canning River estuary in Western Australia in 2001 (River Science, 2005). There is no record of these blooms in America, which motivated the interest in studying the bloom in a small water body in Mexico City.
Our planet is in the Anthropocene era, threatening biodiversity, and human life. Studying fast-growing, oxygen-producing, nutraceuticals and biodisel producing microalgae could help combat climate change and greenhouse gases. Chlamydomonas reinhardtii is a species that can be manipulated in artificial cultivation to produce high-value products such as biodiesel, nutraceutical, food, and medicinal substances. In addition, this alga has biotechnological potential since it grows in small spaces, without competition for space, by taking advantage of arid soils for its cultivation, as well as sunlight, which together with the physical and chemical conditions of the water could promote rapid and optimal growth (Scranton et al., 2015).
This research’s objective was to determine the species forming the bloom observed in 2016 in the North Lake of the Cantera Oriente and its morphological and chemical characterization.
MATERIAL AND METHODS
Study area. The bloom occurred in one of the four lakes located within the Cantera Oriente (CO) (19°19′08” N and 99°10′21” W), which is part of the Reserva Ecológica del Pedregal de San Ángel (REPSA), Mexico City, with an area of 206 000 m2. The Cantera Oriente arose due to the extraction of volcanic rock (basalt), a concession of the UNAM to the Mexico City Asphalt Plant from 1970 to 1994; two years later, the Cantera Oriente was incorporated into the REPSA (Lot, 2007).
The climate of the Cantera Oriente is temperate sub-humid, with summer rains and dry winters. The average annual precipitation was 833 mm; the average annual temperature was 15.6 °C; the prevailing winds came from the N and NNE, and the altitude was 2559 m.a.s.l. The lakes in this area are classified as high-altitude aquatic systems; however, they are tropical lakes, considering their latitude. These water bodies are shallow, and despite sharing the same supply source, they present different environmental conditions, especially regarding their trophic status. The water bodies of the Cantera Oriente were characterized as eutrophic-hypertrophic (Lugo-Vázquez et al., 2017).
Due to its geographic location, the lake understudy was named Lago Norte (LN), with a maximum length of 198 m and 77 m in its widest portion, with an approximate area of 5450 m2. It has a narrow channel (~10 m wide x ~159 m long) that is not easy to access, and it is presumed that water flows from the channel and runs into the water table. In the southern region, a gate links the LN to another larger aquatic system, Lago Centro (LC), which feeds water to the LN along with other channels from springs in the aquifer. The LN is a shallow body with a maximum depth of 1.20 m and is surrounded by arboreal vegetation, shrubs, and aquatic plants rooted in some parts of the littoral zone (Typha latifolia L.), with Stuckenia pectinata (L.) Börner predominant in the channels and free-floating Lemna gibba L. (Lot, 2007; Cuevas-Madrid et al., 2020).
Light and Confocal Microscopy. Sampling was conducted on February 23, 2016, corresponding to the cold-dry season. Direct samples were taken from the surface layer (5 cm) to determine the morphological characteristics of the microalgae. A Zeiss light microscope (LM), model 1206 S09432 (Germany) was used, and images were taken with a Canon PowerShot G6 digital camera (Japan). For the confocal microscopy study, cells were fixed using 2% glutaraldehyde and re-suspended in a phosphate solution (pH 7.2-7.4, 0.1 M) (Mohan, 2006) and stained with Nile red (Sigma, Saint Louis, USA) at a final concentration of 2.5 µg mL-1 (from a stock solution of 50 µg mL-1 in methanol), followed by a 10 min incubation in the dark (Greenspan et al., 1985; Siaut et al., 2011). Images were captured using a Zeiss LSM (Germany) 800 confocal microscope with DIC optics and a plan-apo-63X oil immersion objective. Post-acquisition image management was performed using Zen software (Carl Zeiss, Germany). Cells were stained with the Nile red and visualized with 488 nm argon laser-generated fluorescence combined with a 560 to 615 nm filter to detect neutral lipids; for the combined Chlorophyll-Nile red fluorescence of polar lipids, a 647 to 753 nm filter was used.
Electron Microscopy. For scanning electron microscopy (SEM), the sample was fixed in glutaraldehyde (2%), washed with distilled water, and dehydrated in alcohols gradually (10-100 %), brought to a critical point (EMITECH K 850), and then mounted in aluminum sample holders on carbon tape and metalized with gold for 2 min (QUORUM Q150R ES). The samples were then observed in SEM (Hitachi SU1510, Hitachi, Japan), at a working distance of 15 mm and a voltage of 15 kV.
For transmission electron microscopy (TEM), the sample was fixed in 2% glutaraldehyde in phosphate buffer (pH 7.2-7.4) for two hours at 4°C. Cells were centrifuged at 500 g (1500 rpm) for 10 min. Post-fixation was performed with osmium tetroxide (1%) for 1 min. They were dehydrated with increasing series (10-100%) of ethyl alcohol at 4°C and embedded in epoxy resin (EPON®). Ultra-sections were 90 nm and contrasted with uranyl acetate (0.5 % to 3 %) and lead citrate (3%). Samples were observed and imaged on a TEM (JEOL, Model JEM1200 EX II, Tokyo, Japan) (Siaut et al., 2011), with a resolution of 0.15 nm, an accelerating voltage of 60 to 120 kV, and a magnification of 50x to 500,000x (Toledo et al., 2016). The service of the Imaging Unit of the Institute of Cell Physiology, UNAM, was used.
Liquid samples and permanent preparations were incorporated with their identification number (NI: 2481, 2486, 2491, 2492, 2510, 2581) in the National Herbarium of Mexico (MEXU).
Chemical analyses. An abundant filtered surface water sample (10L) of Chlamydomonas was lyophilized for chemical analyses. According to the methods proposed by AOAC International (2019), lipid content was determined by Soxhlet extraction (AOAC:920.85), nitrogen by Kjeldahl method (AOAC:978.04), protein percentage was calculated as the percentage of nitrogen multiplied by the factor 6.25, and ash content by combustion in a muffle at 550 °C. (AOAC:930.05). Total carbohydrate content was determined by difference [100 - (%moisture + %ash + %fat + %protein + dietary fiber)] (Pehrsson et al., 2015). Dietary fiber (AOAC:992.16) was quantified with enzymatic (α-amylase 5%) and gravimetric autoclave treatment, and the residue was weighed to determine the ratio. Moisture was determined by vacuum drying (3 h) at a constant temperature (70°C) (AOAC: 925.04).
A homogenized sample of the algae was extracted using the methanol/dichloromethane method (2v/1v) to ascertain the fatty acid composition. Methyl esters (FAMEs) were prepared according to AOAC Method (AOAC:996.06). Fatty acids determination was performed with an FID gas chromatograph (Scion Model 436-GC, Compass CDS Software Version 3.0.2.144), equipped with a flame ionization detector and a capillary column (Thermo Scientific TG-Polar 105 m*0.25 mm * 0.2 μm). Ultrahigh purity nitrogen was used as the carrier gas, and the method used was: 100 °C for 4 min, a gradient of 3 °C min-1 to reach 240 °C for 15 min. The chromatograms were identified with a FAME mixture standard’s retention times and correction factors (CRM47885, Supelco).
The catalytic combustion technique analized the percentage of total de carbon at 900°C in a Shimadzu brand equipment model TOC-L SSM-5000A (Shimadzu Corp., Kyoto, Japan; Jha et al., 2014). For total nitrogen, digestion with H2SO4 and vapor entrainment titration was carried out. Total phosphorus was quantified by molybdovanadate yellow complex colorimetry (AOAC: 995.11).
Cell density, chlorophyll, and environmental parameters. Direct samples were taken in triplicate from the surface using Falcon tubes (50 mL). From each sample, three counts were performed to obtain cell density (n=9), using a Neubauer camera and 40X magnification.
These same samples were used to quantify chlorophyll-a concentration by performing triplicate measurements (n=9). A known volume (1 ml) was filtered, using Whatman GF/F fiberglass filters, according to the cold extraction method (4ºC) with 90% acetone, using a Turner Designs model 10-AU fluorometer (Arar & Collins, 1997).
The main environmental parameters of the water column were measured in situ with conventional methods, using a YSI model 85 multiprobe and pH with a Conductronic model pH10 field potentiometer.
Chemical digestion of the organic matter was previously carried out with potassium persulfate solution (100 ml 0.375 N NaOH, 5 g of Potassium Persulfate, and 3 g of Boric Acid) under alkaline conditions using Valderrama (1981) method to quantify total phosphorus and nitrogen. Then, total nitrogen was measured as N-NO3-1 and total phosphorus as P-PO4+3. The concentrations of ammoniacal nitrogen N-NH3, nitrogen as nitrites (N-NO2) and nitrogen as nitrates (N-NO3), and dissolved reactive phosphorus (P-PO4) were also measured. Dissolved inorganic nitrogen (DIN) was calculated from the above data. Bicarbonate concentration was calculated using the proportion between phenolphthalein and methyl orange alkalinity and transforming HCO3-1 concentration as mg L-1 as CaCO3 in HCO3 (mg L-1). Concentrations of Ca+2 and Mg+2 was calculated using the total and calcium hardness data. Methods described by APHA et al. (1985) were used.
Statistical analyses. A linear regression analysis between cell densities and chlorophyll-a concentration was performed using the PAST 4.06 package (Hammer et al., 2001). Both data set were previously transformed to log10.
RESULTS
Taxonomy and morphology. Chlamydomonas is classified within the Phylum Chlorophyta, Class Chlorophyceae, Order Chlamydomonadales, and Family Chlamydomonadaceae. It is a unicellular alga that is distinguished into different subgenera depending on the position of the pyrenoid. In the case of the Cantera Oriente bloom, the alga has a basal pyrenoid that includes it in the subgenus Euchlamydomonas and the Bivacuolatae group because it presents two contractile vacuoles apically. Blooming species was determined as Chlamydomonas reinhardtii P.A. Dangeard, 1888 (Fig. 1B).

Figure 1 Blooming of Chlamydomonas in the Cantera Oriente lake (February 23, 2016). (A) Appearance of the algae on the surface of the lake. (B) Massive appearance of the algae in microscopic view.
Light microscopy (vegetative cells). Cells are solitary, free, broadly ovoid, 8-30 μm long, and 7-22 μm in diameter (Fig. 2A, B). Cup-shaped chloroplast and single pyrenoid, with a halo of starch grains, located in the lower part of the chloroplast (Fig. 2B). Two flagella of equal length or slightly shorter than the cell body emerges from the cell’s anterior region without a papilla (Fig. 2C). The protoplast is observed separately from the cell wall (Fig. 2B, E). The base of the cell is circular 7-23 μm; in this view, the pyrenoid is observed to be large and well-positioned in the central part (Fig. 2D).
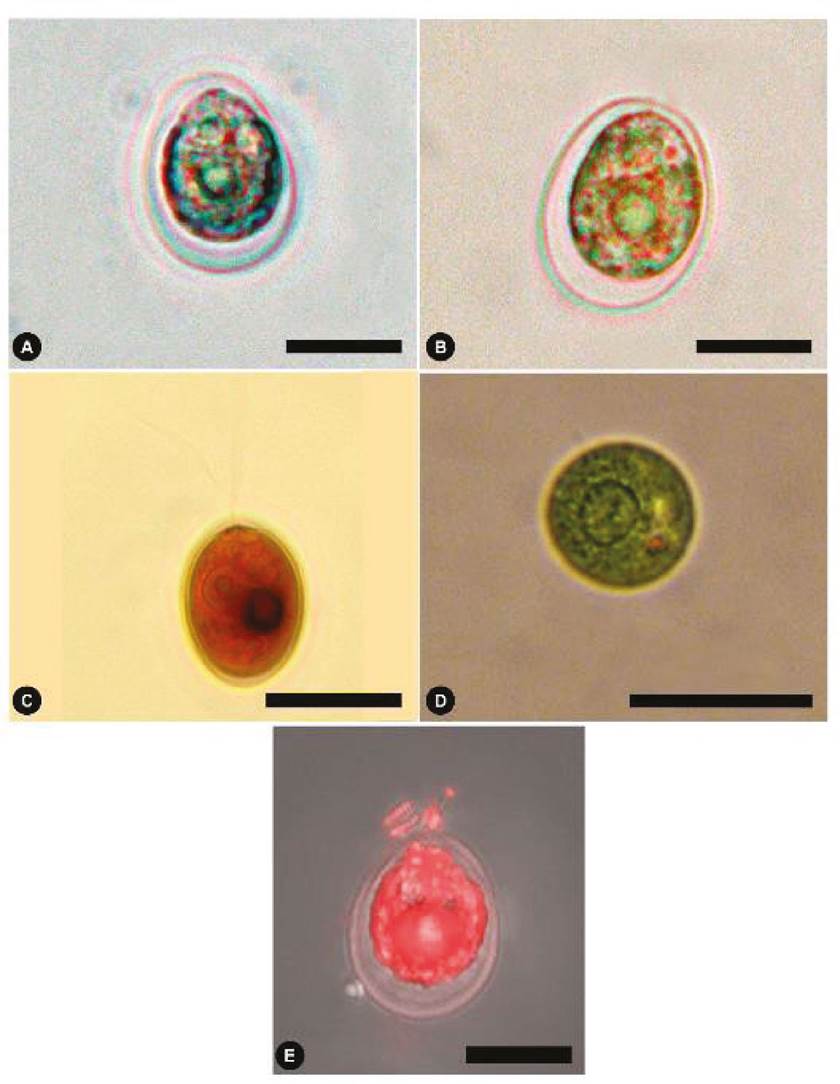
Figure 2 Chlamydomonas reinhardtii from the Cantera Oriente bloom. (A-B) Light microscopic views of the alga. (C) View of cell with two flagella, stained with Lugol. (D) Basal view of living alga, central pyrenoid is observed. (E) Cell with Nile red staining in fluorescence. Scale bars represent: A-C, 5 µm; D, 10 µm; E, 20 µm.
Scanning and transmission electron microscopy (vegetative cells). The cells are ovoid or truncated pear-shaped, without a papilla in the longitudinal section (Fig. 3A). Remnants of the flagellar base are visible in the anterior region, just above the vacuoles. Mitochondria were located near the anterior-lateral cytoplasm under two contractile vacuoles, the stigma and laterally the chloroplast with thylakoids (Fig. 3B). The nucleus is spherical (Fig. 3A, B) and situated slightly above the medial region. The cup-shaped chloroplast contains a pyrenoid in the posterior region (Fig. 3A, C). In the basal view, the pyrenoid is centrally located (Fig. 3C) with a white halo containing starch (Fig. 3D); tubular structures are observed in the centre of the pyrenoid (Fig. 3C, D). The relatively large stigma is located above the mitochondrion in the lateral-anterior region (Fig. 3B) above the mitochondrion.

Figure 3 TEM and SEM micrographs of Chlamydomonas reinhardtii. (A) TEM overview of a complete cell. (B) Detail in TEM with mitochondria and stigma. (C) TEM basal view, pyrenoid with starch granules. (D) TEM detail with a pyrenoid with microtubules. (E) SEM view of complete cell and a vestige of the anterior flagellum. (F) Basal view in SEM. N, nucleus; CP, chloroplast; V, vacuoles; FB, flagellar base; M, mitochondrion; T, thylakoids; P, pyrenoid; S, starch; MT, microtubules; E, stigma. Scale bars represent: A, C, 2 µm; B,0.5 µm; D, 1 µm; E, F, 10 µm.
In SEM, the cells in the lateral view are flattened pear-shaped with a smooth wall and a vestige of the flagellar apparatus in anterior position (Fig. 3E); in the basal view, the cells are circular and smooth-walled (Fig. 3F).
Table 1 summarizes the morphological characteristics of C. reinhardtii, including habitat and geographic distribution. The species under study is compared with four other microalgal species. C. reinhardtii is the only species (Table 1) to be ovoid; in ML, its anterior region is ellipsoid, with a large basal pyrenoid and a mature thick-walled zygospore with anastomosing thickenings. The habitat is freshwater with a wide or cosmopolitan distribution, except for arctic regions (Table 1). Some organisms were mixed with Chlamydomonas with very low abundance (Desmodesmus abundans (Kirchner) E.H. Hegewald, diatoms, and some rotifers).
Table 1 Comparison of principal morphological features of Chlamydomonas reinhardtii and other species.
| Feature | C. reinhardtii | C. ehrenbergii | C. snowiae | C. globosa | C. incerta |
| Cell shape | ovoid | pear-shaped | piriformis | spherical | spherical - broadly ellipsoid |
| Cell size | 7-22 x 8-30 µm | 14-26 µm | 4-18 x 10-24 µm | 5-7.8 µm | 8-9.5 x 9.7-12.4 µm |
| Anterior shape | ovoid | slightly apiculate or narrow | conical | rounded | rounded |
| Posterior shape | rounded | rounded | rounded, basally thickened | rounded, slightly thickened | rounded |
| Flagella length | slightly shorter as the cell | 1½-2 times body length | slightly shorter as the cell | 1½ times longer as the cell | slightly longer as the cell |
| Position of the nucleus | above the middle of the cell | in the middle of the cell | above the middle of the cell | in the middle of the cell | above the middle of the cell |
| Contractil vauoles | 2 apical | 2 apical | 1 apical | 1 apical | 2 apical |
| Cell wall papilla | no | yes | conical | no | no |
| Cell wall | smooth | smooth | with a series of fine lateral striations | smooth | smooth |
| Chloroplast shape | cup-shaped | cup-shaped, thickened at the base | cup-shaped | cup-shaped | cup-shaped |
| Pyrenoid | 1, round-slightly | 1 small, round | 1 | small, round-sligthtly ellipsoid | mediµm, round-slightly ellipsoid |
| Position of the pyrenoid | basal | eccentric | basal | basal | basal |
| Eyespot | 1 elliptic | 1 elliptic | 1 or more | 1 dot-like | elliptic |
| Position of the eyespot | anterior side | median or anterio sider | median or anterio side | median or anterior side | anterior side |
| Plasma membrane | oftenly withdrawn from the cell wall | protoplast sometimes detached from cell wall | no | no | no |
| Zygospore wall | smooth with hexagonal wall thickenings | thick, pitted, and sculpted outer layer (fine warts). | unknown | unknown | unknown |
| Zygospore size | 21.1-33 µm | 12-16 µm | |||
| Habitat* | freshwater | freshwater | freshwater/terrestrial | freshwater/terrestrial | freshwater |
| Distribution* | Europe, North America (Mexico), South America, Middle East, South-west Asia, Asia, Australia, New Zealand | Africa, Europe, Middle East, North America, South America, South-west Asia | Arctic, Europe, North America, South America, Middle East, South-west Asia, Asia, Australia, New Zealand | Europe, North America, South America, Middle East, South-west Asia, Asia, Australia, New Zealand | Europe, North America, Caribbean Islands, Middle East, Asia |
| Reference | This study | Pascher (1927); John et al., (2011) | John et al. (2011) | John et al. (2011); Pröschold et al. (2018) | Pröschold et al. (2018) |
Light and electron microscopy (reproductive cells). Figures 4A-C show different stages of zygospores (juvenile to mature), with a diameter that ranged from 20 to 33 μm. Using Nile red staining and fluorescence in LM, bright yellow lipid droplets are distinguishable within the zygospore (Fig. 4A). Figures 4A and 4B show a thick and smooth outer wall and a thin inner membrane; under the wall, there are wavy or honeycombed ornamentations (Fig. 4A, B); the cytoplasm had a positive reaction to Lugol due to the abundance of starch; other developing reproductive cells without a reticulated wall are also observed (Fig. 4B). In SEM, an anastomosing reticulum protruding from the hexagonal-shaped outer layer is observed (Fig. 4C). In TEM, a well-formed two layers’ wall is observed; a darker outer one and a more homogeneous inner one; undulations in the outer layer can be distinguished that coincide with the anastomosed reticular thickenings; inside the cell, a homogeneous cytoplasm without the presence of organelles is observed, with many white granules, which probably contained reserve substances (Fig. 4D).

Figure 4 Zygospores of Chlamydomonas reinhardtii from the Cantera Oriente bloom. (A) Bright yellow lipid granules with Nile red staining in fluorescence. (B) Zygospores at different stages of development with Lugol stain, (C) Zygospore in SEM; (D) Zygospore in TEM. Scale bars represent: A-C, 10 µm; D, 2 µm.
Chemical analyses. The chemical composition of a wild population of C. reinhardtii during bloom in February 2016 is presented for the first time and compared with other microalgae grown under laboratory conditions (Table 2). Globally, chemically studied microalgae are represented by only ten species; however, based on these studies, we observe that the protein values of C. reinhardtii are in the concentration range of these microalgae (11-77 %, Barka & Blecker, 2016); carbohydrates (10-57%, Becker, 2004) and lipids (4-22 %, Becker, 2004). Currently, considering algae as an essential protein source is based not only on its high concentration but also on the set of other chemicals such as carbohydrates, fats, antioxidants, vitamins, etc. (Becker, 2004). The carbohydrate:protein ratio was low (0.15), while the lipid:protein ratio was slightly higher (0.3).
Table 2 Comparative chemical analysis of Chlamydomonas reinhardtii.
| Relative contents (%) | Phylum | Origin | Protein | Carbohydrate | Lipid | Ash | Reference |
|---|---|---|---|---|---|---|---|
| Chlamydomonas reinhardtii | Chlorophyta | * | 19.2 | 2.9 | 6.55 | 25.5 | This study |
| Chlamydomonas reinhardtii | Chlorophyta | ** | 46.9 | 23.0 | 24.7 | 4.8 | Darwish et al. (2020) |
| Chlorella sp. | Chlorophyta | ** | 45.3 | 28.0 | 16.1 | 9.3 | Darwish et al. (2020) |
| Dunaliella salina | Chlorophyta | ** | 22.4 | 21.7 | 3.0 | 52.8 | El-Baz et al. (2017) |
| Tetradesmus obliquus | Chlorophyta | ** | 19.0 | 68.8 | 11.0 | 1.1 | Oliveira et al. (2020) |
| Limnospira maxima | Cyanobacteria | ** | 50.4 | 23.0 | 14.1 | 11.4 | Darwish et al. (2020) |
| Anabaena cylindrica | Cyanobacteria | ** | 43-56 | 25-30 | 4-7 | 5-10 | Becker (2004); Allen & Arnon (1955) |
| Synechococcus sp. | Cyanobacteria | ** | 63 | 15 | 11 | Becker (2004) | |
| Porphyridium cruentum | Rhodophyta | ** | 0.07-0.34 | 72.6-77.7 | undetected | 14-32 | Agustina et al. (2020) |
* Wild algae; ** Cultivation conditions
Fatty acid percentages are presented in Table 3. In C. reinhardtii from the Cantera Oriente, saturated fatty acids (SAFA, C16:0, 3.7%) were in higher proportion than monounsaturated (MUFA, 0.8%) and polyunsaturated (PUFA, 0.2-0.6 %). Palmitic acid (3.7%) stands out, followed by oleic acid (0.8%) and then linoleic acid (0.6%).
Table 3 Fatty acid profile of Chlamydomonas reinhardtii (%).
| Fatty acids | Common name | C. reinhardtii* | C. reinhardtii** | C. reinhardtii** | Tetradesmus obliquus** | Chlorella sp.** | Dunaliella salina** | Limnospira máxima** |
| C12:0 | Lauric acid | ND | - | - | 0.3 | - | - | - |
| C14:0 | Myristic acid | 0.7 | - | 0.43 | 0.6 | - | 0.9 | - |
| C16:0 | Palmitic acid | 3.7 | 23.8 | 28 | 16 | 22.2 | 6.0 | 57.9 |
| C16:1ω7 | Palmitoleic acid | ND | 27.0 | - | 8 | 13.0 | 0.8 | 0.1 |
| C18:0 | Stearic acid | ND | 23.0 | 4.4 | 0.3 | 28.0 | 31.5 | 1.5 |
| C18:1ω7 | Oleic acid | 0.8 | - | 4.89 | 8 | - | 5.9 | - |
| C18:2ω6 | Linolenic acid | 0.6 | 3.8 | 12.1 | 6 | 31.4 | 0.8 | 19.0 |
| C18:3ω3 | α-linolenic acid Omega 3 | 0.2 | 4.1 | 9.77 | 28 | 23.4 | - | 0.1 |
| C20:0 | Arachidic acid | 0.5 | - | - | - | - | 0.1 | - |
| Reference | This study | Darwish et al. (2020) | Ochoa-Alfaro et al. (2019) (pH 7.8) | Becker (2004) | Darwish et al. (2020) | El-Baz et al. (2017) | Darwish et al. (2020) |
* Wild; ** Cultivation condition
Neutral lipids (triacylglycerols) were observed in reproductive cells (zygospores), using the Nile red fluorescence technique, like bright yellow circular droplets (Fig. 4A); conversely, vegetative cells showed no reaction to the Nile red (Fig. 2E). Ash values are well within the wide range of microalgae (8-40%, Table 2) (Becker, 2004).
Element contents (C, N, P) are presented in Table 4. Algal carbon (37.37%) was higher than nitrogen (3.26%) and phosphorus (0.44%). The C/N ratio was higher (11.46%) than the N/P ratio (7.40%), and the C/P ratio was high (84.93%).
Table 4 Nitrogen, phosphorous and carbon content of Chlamydomonas reinhardtii bloom.
| Element | % | SD |
| N | 3.26 | |
| P | 0.44 | |
| C | 37.37 | 0.44 |
| C/N | 11.46 | 0.13 |
| N/P | 7.40 | |
| C/P | 84.93 |
Cell density, chlorophyll, and environmental conditions. Cell abundance or cell density values were high (6.98 x 10-5 ± 1.37 x 10-5 cells mL-1), which is confirmed by extreme values of chlorophyll-a concentration (5548 ± 796 µg L-1) (Fig. 5). Regression analysis between the log of Chlamydomonas cell abundance values and the log of chlorophyll a concentration was significant (r2= 0.9362, p<0.001). Residuals have a normal distribution (Shapiro-Wilks test 0.8995, p= 0.272), and the Durbin-Watson test (2.9995, p=0.95) indicated no significative autocorrelation in y values residuals. The Breush-Pagan statistic (1.4645) corroborates the homoskedasticity (p=0.226) of residuals. Data used for regression analysis were obtained directly from field samples. This fact explained the not-so-high r2 value obtained. Several other phytoplankton species could be present in variable abundances in the samples, increasing the chlorophyll concentrations and reducing the relationship between both variables.
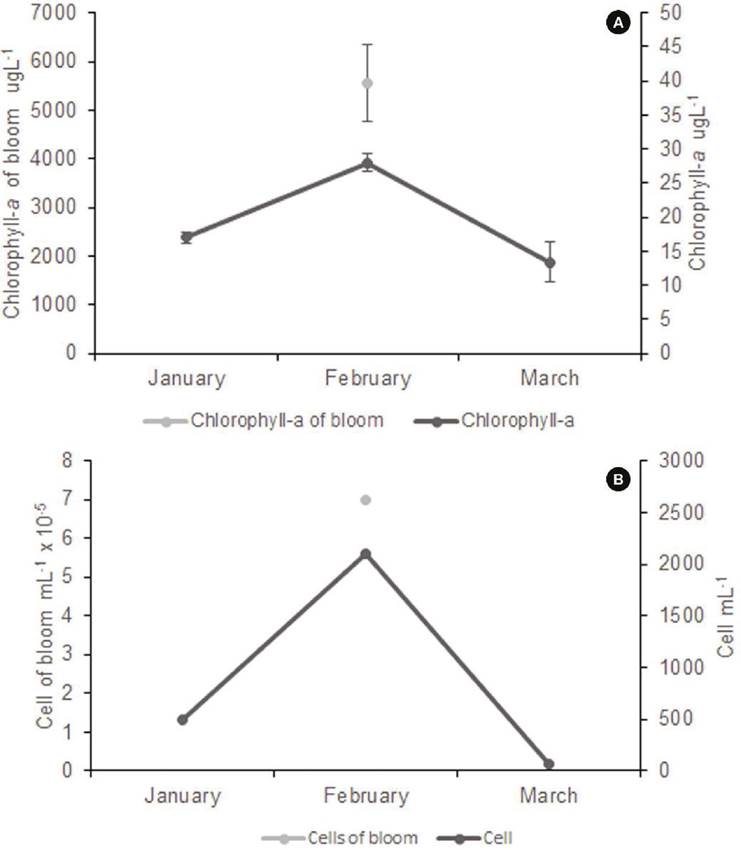
Figure 5 Chlorophyll concentration (A) and Chlamydomonas cell density (B) before (January 2016), during (February 2016), and after bloom (March 2016).
Photosynthetically active light was recorded in the study period (January-March) in a range of 0.003-2361 µmol photon m2 s-1 (Fig. 6); an increase was observed towards March.

Figure 6 Photosynthetically active radiation (PAR) before (January 2016), during (February 2016), and after bloom (March 2016).
The environmental conditions during the bloom do not allow us to identify the variables promoting the bloom. The blooming occurred during the coldest period of the year, with temperatures around 15 °C. Several variables showed a slight decrease during bloom, such as the concentration of bicarbonate (39 to 34 mg L-1), calcium (37 to 34 mg L-1), and nitrates (5.9 to 5.4 mg L-1). Others also increased slightly, such as dissolved reactive phosphorus (0.11 to 0.15 mg L-1), nitrogen as nitrite, N-NO2 (0.013 to 0.021 mg L-1), magnesium (26 to 29 mg L-1) and conductivity (389 to 419 µS cm-1). The N-NH3 concentration doubled (0.05 to 0.10 mg L-1). Finally, variables whose values increased probably due to the bloom effect, such as D.O. (8.1 to 8.8 mg L-1) and pH (6.7 to 7.3). Table 5
Table 5 Environmental conditions in the North Lake before (January 2016), during (February 2016), and after the algae blooming (March 2016) of C. reinhardtii.
| Parameter | Before algae blooming | During algae blooming | After algae blooming |
| Temperature (° C) | 12.1 | 14.7 | 17.0 |
| pH | 6.7 | 7.3 | 7.4 |
| Bicarbonate (mg L-1) | 39 | 34 | 34 |
| Calcium (mg L-1) | 37 | 34 | 32 |
| Magnesium (mg L-1) | 26 | 29 | 25 |
| Conductivity (µS cm-1) | 389 | 419 | 392 |
| D.O. (mg L-1) | 8.1 | 8.8 | 7.1 |
| P-PO4 (mg L-1) | 0.11 | 0.15 | 0.15 |
| N-NH3 (mg L-1) | 0.05 | 0.10 | 0.18 |
| N-NO2 (mg L-1) | 0.013 | 0.021 | 0.042 |
| N-NO3 (mg L-1) | 5.9 | 5.4 | 5.6 |
DISCUSSION
Because of the basal position of the pyrenoid and a cup-shaped chloroplast in both ML and TEM microscopy, this alga is included in the subgenus Euchlamydomonas. The specific diagnosis was C. reinhardtii, based on cells with two apically contractile vacuoles oriented perpendicular to the plane of the two flagella (Bivacuolatae group); the cell has a smooth chloroplast, without divisions or lobules; the cells are neither hemispherical nor flattened, the stigma is present, the cell is broadly ovate and has no papilla. It is closely related to C. ehrenbergii, due to its size, shape, ultracellular arrangement, and the presence of two vacuoles below the flagellar apparatus; however, C. ehrenbergii is apiculate in its anterior region and has a papilla, as does C. snowiae. It was observed that the species under study has a plasma membrane that separates from the cell wall, which is not the case with C. globosa, C. snowiae and C. incerta (Table 1); in addition, C. globosa is smaller (5-7.8 µm), spherical, and has only one contractile vacuole. C. incerta differs from C. reinhardtii by containing flagella larger than its cell and is spherical to ellipsoidal (Table 1). The zygospores of C. reinhardtii differ from C. ehrenbergii in size; the outer, mature cell wall has anastomosing thickening in C. reinhardtii and warty in C. ehrenbergii. The zygospore seen in TEM sections presents a two-layered cell wall, which is very similar to those of Heimerl et al. (2018), who studied the same species.
Regarding the chemical composition of the blooming algae, it had a moderate protein content (19.2 %). Differences are observed in comparing the protein, carbohydrate, and lipid content of C. reinhardtii with other microalgae; the cyanobacterium Limnospira maxima had the highest protein concentration (50.4 %), as did other green algae such as Chlorella sp. (45.3 %). The cultivated strain of C. reinhardtii also had a higher protein content (46.9 %). The Mexican strain presented a low amount of carbohydrates (2.9 %) compared to the cultured microalgae Porphyridium cruentum (72.6-77.7 %), C. reinhardtii (23.0 %), Chlorella sp. (28.0 %), and Tetradesmus obliquus (68.8 %). The lipids of C. reinhardtii (6.5 %) from the Cantera Oriente had similar values to Anabaena cylindrica (4-7 %) and were in higher proportion than Dunaliella salina (3.0 %); but their values were lower than cultured C. reinhardtii (24.7 %), Tetradesmus obliquus (11.0 %), and Chlorella sp. (16.1 %). The analysis showed a high proportion of ash (25.5 %) compared to Chlorella (9.3 %) and Limnospira (11.4 %). In general, the species under study presented marked poverty of fatty acids compared to other species (Table 3); however, myristic acid (0.9 %), linolenic acid (0.8 %), arachidonic acid (0.1 %) of D. salina, and a-linolenic acid (Omega-3) of L. maxima had similar values with C. reinhardtii from the Cantera Oriente. In contrast, linolenic acid from Chlorella sp. (31.4 %), L. maxima (19.0 %) y C. reinhardtii (12.1 %), presented higher concentrations than in wild C. reinhardtii (0.6 %). However, it must be considered that the algae under study are from wild or natural conditions, and the other algae, such as Chlorella, Limnospira, Tetradesmus, and Dunaliella (Tables 2, 3), come from controlled cultures that could promote an increase in these substances.
The absence of intracellular lipid bodies through the Nile red reaction and fluorescence (Fig. 2E) and the absence of grey nodules (fat droplets) in transmission microscopy photography lateral and basal view respectively (Fig. 3A, C) confirmed that triacylglycerols (TAGs) were absent. In contrast, both storage entities (starch granules and lipid bodies) were found abundant in 30-day cultured cells in laboratory cultures (Darwish et al., 2020). It is well known that lipid accumulation as TAG in microalgae increases as cells age, usually due to intracellular nutrient (nitrate) consumption during stationary growth and is accompanied by a cessation in cell division. Salas-Montantes et al. (2018) points out that the biosynthesis of triacylglycerols from algae is of growing interest for biodiesel production. They found that changes in gene expression in the Chlamydomonas reinhardtii strain overexpressing a transcription factor in response to nutrient deficiency increased its total fatty acid content (17.02%) in a medium without nitrogen. The most common fatty acids found were palmitic, oleic, and linoleic. Ochoa-Alfaro et al. (2019) observed an increase in cell growth, chlorophyll, and lipids concentration at pH values of 7.8. The authors point out that combining different strategies can help to obtain a high-value product (Hernández-Torres et al., 2016).
This reflects that the Cantera Oriente bloom was not in a stationary phase, but in a state of exponential growth and cell division, efficiently utilizing nutrients (Khozin-Goldberg & Cohen, 2006; Moellering & Benning, 2010). Lipid droplets were only observed in the resistance structures (zygospores), meaning that these structures require lipid storage to enter dormancy. Zygospores were very scarce.
Chlorophyll-a concentration was high in the bloom (5548 µg L-1) compared to the natural concentration in the water column in January and March (17.05 and 13.38 µg L-1). Chlorophyll-a concentration is an indicator of algal biomass. It is related to the fact that during the bloom, there is more protein production because the carbohydrate:protein ratio (0.15) was very similar to that reported in an experimental culture of C. reinhardtii (0.13 to 1.2) during the exponential phase (Dean et al., 2008). The biochemical relationship of carbon, nitrogen, and phosphorus content to producing organic compounds is a necessary and survival relationship (Moellering & Benning, 2010). The N:P ratio is around 16 when in a stoichiometric equilibrium state, while in the Cantera Oriente an N:P ratio of 43 was obtained which exceeded the Redfield ratio, indicating a low proportion of phosphorus (Granéli et al., 2008; Smith et al., 2017). The three primary fatty acids (Omega-3) contained in the membranes are alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). Given the presence in the alga understudy of ALA (Table 3) in chloroplast membrane glycerolipids, its importance in maintaining membrane fluidity at optimal growth temperatures (Hixson & Arts, 2016) and preventing membrane rigidity at suboptimal low temperatures can be assumed (Mansilla et al., 2004). Phosphate availability in the plant cell is often limited, and galactolipids predominate in plants and algae to reduce algal dependence on phosphate (Kalisch et al., 2016). It has been seen that with severe phosphate limitation, chlorophyll synthesis is delayed but not stalled, as intracellular phosphate is sufficient to continue cell division, at least for a short time (Khozin-Goldberg & Cohen, 2006). There was a trend towards phosphorus limitation in the dry season in the NL, but total P deprivation for algae never occurred. This is proven by seeing phosphate-deprived microalgae with an increase in triacylglycerols (TAG), observed as oil droplets in the cells, which was not detected in the Chlamydomonas from the Cantera Oriente (Khozin-Goldberg & Cohen, 2006). The substantial imbalance found in the N:P ratio (Table 4) demonstrates physiological stress with the possibility of increased production of allelochemical compounds resulting in Chlamydomonas developing as a mono-specific population (Granéli et al., 2008).
Ash correlates directly with the aquatic environment’s concentrations of inorganic substances and salts. According to Darwish et al. (2020), the relatively high ash content may help explain its high pigment concentration because chlorophyll has an inorganic magnesium core.
The total carbon content of the alga is related to the carbohydrate content (Table 4, in Fig. 3C, D; Fig. 4B). During photosynthesis, phytoplankton absorbs carbon dioxide from the environment and trap solar energy, using this energy to convert inorganic carbon into carbohydrates and releasing oxygen to the environment (Reynolds, 1984; Basu & Pick, 1997; Altman & Paerl, 2012). The presence of starch is very evident in the reaction to Lugol (Fig. 2C) and in the starch halo surrounding the pyrenoids (Fig. 3C, D).
There has been much debate about the concentrations of nitrogen and phosphorus needed to cause algal blooms in water bodies. Algal response to nitrogen and phosphorus differs among aquatic ecosystems, and the development of notable phytoplankton blooms may require lower concentrations of soluble inorganic phosphorus (0.01 to 0.1 mg L-1) and inorganic nitrogen (0.1 to 0.75 mg L-1) (Boyd, 2015). These bloom-development conditions at low phosphorus concentrations were observed in the case of the Cantera Oriente.
The linear regression model showed that chlorophyll-a concentration was positively correlated with abundance (p < 0.001; r2 = 0.94), indicating that there seems to be a significant contribution of Chlamydomonas to primary production during bloom through the photosynthesis they can perform with the concentration of chlorophyll-a they contain. The values of chlorophyll-a abundance and concentration are very high, but it must be considered that the bloom occupied only a few superficial centimeters of the water column, which moderates its contribution to production.
Water quality is influenced by geological, hydrological, climatic, and anthropogenic factors (Espinal-Carreón et al., 2013; Boyd, 2015); however, water temperature is considered one of the most influential parameters in water bodies (Wetzel & Likens, 2000). The Chlamydomonas bloom in the northern lake occurred in winter when the water temperature was slightly lower; however, Janse van Vuuren & Pieterse (2005) noted that Chlorophyceae (green algae) could occupy a more comprehensive temperature range, from below 15 °C to above 20 °C (Hixson & Arts, 2016).
It is concluded that the algal bloom consisted mainly of Chlamydomonas reinhardtii determined based on LM, SEM, TEM, and fluorescence studies. However, it would be essential to perform molecular studies of this Mexican species in the future. The presence of intracellular oil droplets (TAG) was not found, indicating that the alga was not in a nutrient-limited state but had active cell division. The abundance of cells in this bloom significantly correlated correlation with the chlorophyll-a concentration, indicating this alga’s high photosynthetic capacity. Chemical analyses of the algae indicated low concentrations of proteins and carbohydrates, although moderate concentrations of lipids. The fatty acid composition was poor but included unsaturated fatty acids of importance in animal feed.
The lipids and fatty acids of C. reinhardtii are scarce compared to cultivated algae (Table 3); however, it is important to mention that this wild alga comes from natural conditions and contains a beneficial alpha-linolenic acid (omega 3), a component in the cardiovascular and neurological health of humans (Darwish et al., 2020). However, further studies of this alga under controlled culture conditions (bioreactor) and its gene expression are required to use it as a biotechnological resource.











 nueva página del texto (beta)
nueva página del texto (beta)



