INTRODUCTION
Seaweed liquid extracts have been used in agriculture for centuries as a fertilizer. Currently, studies on seaweed liquid extracts have reported that they have plant growth-promoting activity, therefore, their use in agriculture and horticulture has increased as foliar sprays for various crops such as grasses, cereals, flower species and vegetables; as well as organic manures and fertilizers (Zamani et al., 2013; Battacharyya et al., 2015; Ertani et al., 2018). These extracts contain essential minerals, proteins, polysaccharides, phytohormones, and low molecular weight compounds that stimulate the plant growth and development (Van Oosten et al., 2017; Ali et al., 2019; Mukherjee & Patel, 2020). The set of these compounds can work synergistically, hence understanding their mechanism of action is highly intricate, due the multiple interaction between the substantial numbers of bioactive compound within the same extract (Zamani et al., 2013; EL Boukhari, 2020).
Particularly, the polysaccharides and their oligosaccharides present in seaweed cell walls may directly affect different physiological processes in plants that are related to growth and development (Salachna et al., 2018; Iwasaki & Matsubara, 2000; Mukherjee & Patel, 2020). These oligosaccharides can induce defence responses against potential pests, pathogens (Klarzynski et al., 2003; Falcón & Cabrera, 2007; Mzibra et al., 2018), and abiotic stress (Salachna et al., 2018). In addition, they have been found that they modulate gene expression and multiple hormones signalling pathways (Rolland et al., 2002; Battacharyya et al., 2015; Ertani et al., 2018).
Seaweed liquid extracts involves the three main groups of macroalgae that are red, brown, and green, however, brown seaweeds are the most used commercially to produce liquid or powder extracts, due to their abundance, large distribution and effectiveness. Among the brown seaweeds, the most used are Ascophyllum nodosum (Linnaeus) Le Jolis , Ecklonia maxima (Osbeck) Papenfuss, Macrocystis pyrifera (Linnaeus) C. Agardh, Durvillaea potatorum (Labillardière) Areschoug (Khan et al., 2009), Fucus spp, Laminaria spp., and Turbinaria spp. (Hong et al., 2007; Sharma et al., 2012), however, other seaweeds as Padina gymnospora (Kützing) Sonder, Sargassum liebmannii J. Agardh, and Sargassum johnstonii Setchell & N. L. Gardner are also used (Drobek et al., 2019).
Compounds such as alginate, fucoidan, laminaran and their oligosaccharides present in brown algae, have been shown to have growth-promoting activity (González et al., 2013; Rengasamy et al., 2015). Some studies have shown that alginate oligosaccharides improve the growth of rice and peanut plants in hydroponic cultures (Hien et al., 2000), stimulate the growth of lettuce (Lactuca sativa Linnaeus) and tomato (Lycopersicum esculentum Linnaeus) roots (Iwasaki & Matsubara, 2000; Yabur et al., 2007), increase the biomass in tobacco plants (Laporte et al., 2007); increase shoot and root length of fennel plants (Sarfaraz et al., 2011).
Studies about fucoidan with application in plants showed that enhance protection against pathogens in tobacco plants (Klarzynski et al., 2003; Lapshina et al., 2006). On the other hand, Bouissil et al. (2020) found that fucoidan from Bifurcaria bifurcata R. Ross and Fucus spiralis Linnaeus stimulate the natural defences of the date palm.
For this study, the brown algae Eisenia arborea Areschoug and Sargassum horridum Setchell & N. L. Gardner were selected considering the large abundance in the Baja California Peninsula. The extraction of alginate and fucoidan from these algae was carried out, with the aim of evaluating their effect on seed germination and their growth-stimulating activity on seedling of mung bean (Vigna radiata (L.) Wilczek).
MATERIALS AND METHODS
Sampling. Seaweeds were harvested (n > 30) from wild populations along the coast of the Baja California Peninsula from March to July 2015. Seaweed collection was conducted by divers at low tide. Eisenia arborea was collected from Bahia Magdalena (24° 35’ 00² N-112° 00’ 00² W) and Sargassum horridum from Bahia de La Paz (24° 08’ 32² N-110° 18’ 39² W). The fresh seaweeds were washed with tap water to remove epiphytic organisms and sand. They were sun-dried to 10% moisture content and milled to 40-mesh size (0.38 mm2).
Fucoidan extraction. Twenty-five grams of algae (triplicate) were extracted twice with 350 ml of distilled water at 55 °C and continuous agitation for 2 h. Afterwards, the liquid was recovered by decanting and clarified by centrifugation (1500 ´ g for 15 min; Beckman TJ-6, Palo Alto, CA, USA). Then, 20 mL of 10% CaCl2 was added to precipitate any residual soluble alginate, which was separated by centrifugation. The clarified solution was precipitated with three volumes of ethanol, and crude fucoidan was recovered by centrifugation, dried at 50 °C for 20 h, and stored at -20 °C until analysis. The crude fucoidan content was determined as a percentage of the initial algal dry weight (Muñoz-Ochoa et al., 2009).
Alginate extraction. The residual algal tissue obtained from the fucoidan extraction was used for the alginate extraction. The dried algal tissue was hydrated overnight with 180 mL of 0.1% formaldehyde solution. The algae were treated with 300 mL of HCl at pH 4 and subjected to constant stirring for 15 min. After filtration, the algae were treated with 300 ml of Na2CO3 solution, and the pH was adjusted to 10. After which, the algae were heated to 80 °C in a water bath (Precision Scientific, Chicago, IL, USA) with constant stirring for 2 h. The obtained paste was diluted and filtered under vacuum. The alginate was precipitated with ethanol, and the alginate fibres dried at 50 °C for 12 h. Alginate yield was computed as the percentage of the algal dry weight. Viscosity was measured in 1% alginate solution at 22 °C with a viscometer (Brookfield LVT, Middleboro, MA, USA) at 60 rpm using the appropriate spindle. A second measure of viscosity was carried out after adding sodium hexametaphosphate to sequester the residual calcium (Rodríguez-Montesinos et al., 2008). The alginate gel strength was measured by preparing calcium alginate gels using 1% alginate solution and filling a dialysis membrane (9 cm length ´ 2.9 cm diameter, which was then immersed overnight in a 10% calcium chloride solution. The gels were cut into cylinders (2.9 cm diameter ´ 3 cm length), and the gel strength measured with a TA.XT Plus texturometer (Stable Micro Systems, Godalming, Surrey, UK) programmed to perform a 2-cm penetration over a 5-s period to measure the gel breaking point (Camacho & Hernández-Carmona, 2012).
Spectroscopic characterization of polysaccharides by Fourier-transform infrared spectroscopy (FT-IR). The Fourier-transform infrared spectroscopy (FT-IR) spectra of the polysaccharides were recorded with a spectrophotometer (Perkin Elmer, TWO, Waltham, MA, USA) equipped with an attenuator of total reflectance (ATR). Each spectrum was obtained from the sum of 14 scans in the spectral range of 4000-500 cm−1. The sulphate-total sugars (SO4/CHOH) ratio was determined from the intensity of the transmittance bands at 1040 and 1256 cm−1 (Lijour et al., 1994).
Bioassays of germination and growth under in vitro conditions. Certified mung bean (Vigna radiata) seeds (High Mowing Organic Seeds, Wolcott, VT, USA) with 95% germination, uniform size, colour, and weight were surface-sterilized using a soap solution for 5 min, followed by immersion in a 4% sodium hypochlorite (NaClO) solution for 10 min. They were then each triple-rinsed in sterilized distilled water for 1 min under aseptic conditions. Mung bean germination was carried out as described by Castellanos-Barriga et al. (2017). First, seven groups of 30 mung bean seeds were teste for germination in each of the experimental treatments (fucoidan EA and SH) or (Alginate EA and SH) at different doses of 0.6, 1.2, 2.5, 5, 10 and 20 mg mL‒1 and control without fucoidan, only distilled water that was used to produce the extracts (fucoidans). The seeds were primed for 15 min in 20 mL in each dose of seaweed polysaccharides (alginate or fucoidans) then they were removed from the polysaccharide doses, wrapped in wet filter paper, and incubated at 25 °C in a 16:8 h light: dark cycle. All experiments were conducted in triplicate. Germination was observed daily over an 8-day period, according to the methods detailed by the Association of Official Seed Analysts (AOSA, 2005). The germination percentage (GP) was determined as the number of germinated seeds/total number of seeds ´ 100. Seeds were considered germinated when the radicle protruded more than 2 mm.
The effects of the fucoidan and alginate from E. arborea and S. horridum on the shoot length (from the basal node to the shoot apex), root length (from the basal node to the root tip), and total seedling length (from the root tip to the shoot apex) on 15 days old mung bean seedlings were measured using a Vernier calliper. In addition, mean dry weights were obtained with an electronic balance after oven-drying the seedlings to a constant weight at 50 °C.
Statistical analyses. The data were analysed by a one-way ANOVA and post hoc a least significant differences (LSD) tests after both normality and homogeneity of variance were tested with Shapiro-Wilk and Bartlett tests, respectively. Fucoidan and alginate obtained from brown seaweeds were analysed for their effect on mung bean growth by a one-way ANOVA with the type of algal species and the extract concentration as factors. Statgraphics Centurion XV (The Plains, VA, USA) for Windows (Microsoft Corp., Redmond, WA, USA) was used for all analyses.
RESULTS
The fucoidan yield for E. arborea and S. horridum was 10.8 ± 0.3% and 11.1 ± 0.2% (w/w), respectively, whereas the alginate yield was 14.9 ± 0.3 and 17.3 ± 0.5% (w/w), respectively.
Spectroscopic characterization of polysaccharides by FT-IR. The FT-IR spectra showed typical absorption bands for fucoidans and alginates. The absorption bands around 3200-3400 and 2937-2945 cm‒1 are typical of the O-H stretch vibration and C-H stretch vibration of algal polysaccharides, respectively. Absorption bands between 1603-1616 and 1406-1418 cm‒1 were observed for C=O asymmetric and symmetric carboxyl group oscillations, and the bands around 1125-1130 cm‒1 were typical of pyranose ring and glycosidic bond C-O stretching vibrations (uronic acids; Table 1).
Table 1 The infrared spectroscopy (IR) spectrum data of crude fucoidan and alginate from Eisenia arborea (EA) and Sargassum horridum (SH).
| Fucoidan | Alginate | ||||
| EA | SH | EA | SH | ||
| Wavenumber (cm-1) | Characteristic Peaks | ||||
| 3200-3400 | 3200-3400 | 3200-3400 | 3200-3400 | O-H stretch vibration | |
| 2945 | 2941 | 2937 | 2937 | C-H stretch vibration | |
| N/D | 1725 | N/D | N/D | C-O stretching vibration of O-acetyl groups | |
| 1616 | 1610 | 1603 | 1606 | C= O asymmetric and symmetric oscillation of carboxyl group | |
| 1418 | 1414 | 1404 | 1412 | ||
| 1250 | 1248 | * | * | S-O stretching vibration of sulfate | |
| 1130 | 1128 | 1128 | 1125 | The pyranose ring and glycosidic bond C-O stretching vibrations (uronic acids) | |
| ND | ND | ND | 947 | C-O stretching of uronic acid residues | |
| 850-840 | ND | ND | ND | C-O-S secondary axial sulfate at C-4 of the fucopyranosyl residue | |
| ND | ND | 813-870 | 811-880 | C1-H deformation vibration of b-mannuronic acid residues | |
| 830-20 | 830 | ND | ND | C-O-S secondary equatorial sulfate at C-2 and C-3 position | |
ND = Not detected, * = Not assigned
The fucoidans from E. arborea and S. horridum showed some differences in their chemical structure. The E. arborea fucoidan showed absorption bands of 840-850 cm‒1 for C-O-S, and a secondary axial sulphate at C-4 of the fucopyranosyl residue (Fig. 1A) and the S. horridum fucoidan did not show. On the contrary, the S. horridum fucoidan showed absorption bands at 1725 cm‒1 for the C-O stretching vibration of O-acetyl groups (Fig. 1A, B) and the E. arborea fucoidan did not have. The sulphate-total sugars (SO4/CHOH) ratio was 0.27 and 0.26 for the fucoidans from E. arborea and S. horridum, respectively.
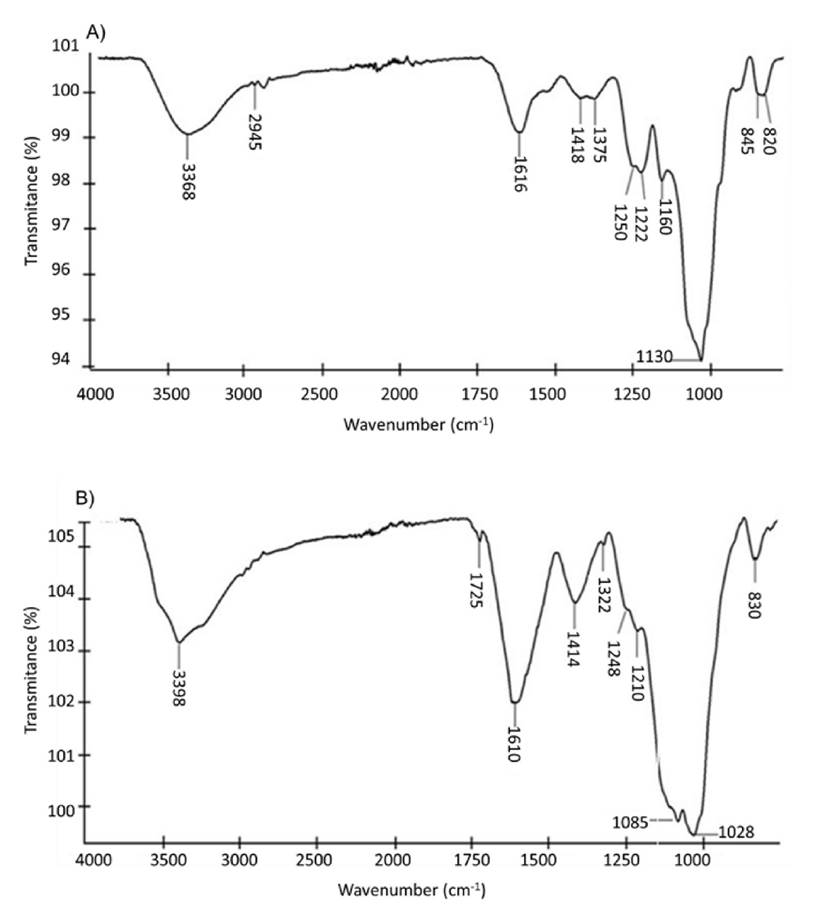
Figure 1 Infrared spectrum (FT-IR ATR) of crude fucoidan from (A) Eisenia arborea and (B) Sargassum horridum.
The alginate infrared spectrum from E. arborea and S. horridum showed the typical absorption bands at 811 and 880 cm‒1 (C1-H deformation vibration of b-mannuronic acid residues) (Fig. 2A, B). However, the S. horridum alginate showed C-O stretching of uronic acid residues evidenced by the absorption band at 947 cm‒1 (Fig. 2B) that the E. arborea alginate did not have.

Figure 2 Infrared spectrum (FT-IR ATR) of alginate from (A) Eisenia arborea and (B) Sargassum horridum.
Germination of mung bean seeds under in vitro conditions. The fucoidan and alginate from E. arborea and S. horridum promoted the germination of mung bean seeds at all doses up to 96 to 100% (Figure 3A, B). The seeds treated with fucoidan, and alginate germinated at 24 h, whereas the control seeds germinated 48 h later.
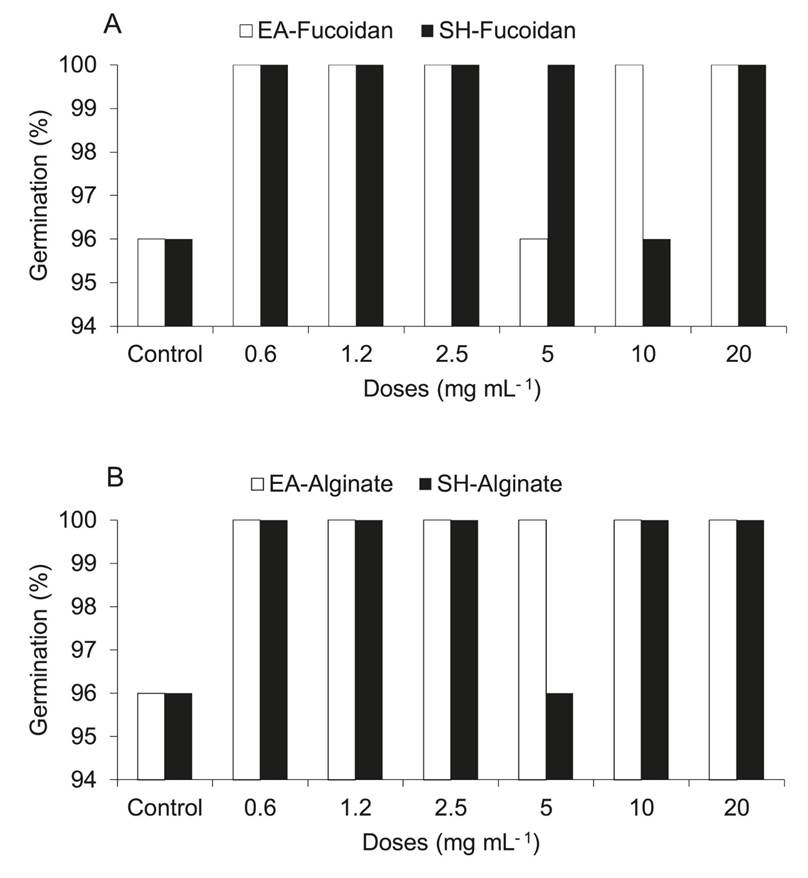
Figure 3 Effect of (A) crude fucoidan and (B) alginates from Eisenia arborea (EA) and Sargassum horridum (SH) at different doses on the germination percentage.
Effect of crude fucoidan and alginate on the growth of mung bean seedlings. A significant difference (p ≤ 0.05) was observed in the growth of mung bean plants treated with crude fucoidans compared to the growth of control plants. E. arborea (EA) fucoidan resulted in an increase in shoot length and total length of 10.5 cm and 20.2 cm respectively, and the greater increase in dry weight of seedling (281.3 mg) with the concentration of 2.5 mg mL‒1 (Figure 4A, C and Table 2). Greater increases in shoot length (10.6 cm) and dry weight (313.7 mg) were found in plants treated with the S. horridum (SH) fucoidan at 20 mg mL‒1 (Figure 4A, D). In addition, the SH-fucoidan at 0.6mg mL-1 showed a significantly higher effect (p ≤ 0.05) on mung bean root length and total length with an increase of 10.4 cm and 20.6 cm, respectively in comparison to control (9.0 cm and 18.4 cm) (Figure 4B, C and Table 2).

Figure 4 Effect of crude fucoidan from Eisenia arborea (EA) and Sargassum horridum (SH) at different doses on (A) shoot, (B) root, (C) total length and (D) Dry weight of mung bean. Columns denoted by asterisk are significantly different to control. Values represent the mean of n = 30 plants; bars represent standard errors.
Table 2 Mung bean growth using different doses of crude fucoidan from Eisenia arborea (EA) and Sargassum horridum (SH).
| Doses (mg mL‒1) | Shoot length (cm) | Root length (cm) | Total length (cm) | Dry weight (mg) |
|---|---|---|---|---|
| Control | 9.4 ± 1.4a | 9.0 ± 2.2a | 18.4 ± 2.9a | 239.4 ± 32.5c |
| EA 0.6 | 10.3 ± 1.7a | 9.5 ± 2.1a | 19.8 ± 2.9a | 237.4 ± 17.6c |
| EA 1.2 | 10.1 ± 1.8a | 8.9 ± 2.5a | 19.0 ± 3.5a | 235.9 ± 9.2b |
| EA 2.5 | 10.5 ± 1.5b | 9.8 ± 3.2a | 20.2 ± 3.8b | 281.3 ± 25.9e |
| EA 5.0 | 9.1 ± 1.6a | 8.6 ± 2.1a | 17.7 ± 2.9a | 224.8 ± 27.0a |
| EA 10 | 10.4 ± 2.2b | 10.0 ± 2.5a | 20.4 ± 3.7b | 241.8 ± 16.1c |
| EA 20 | 10.2 ± 1.9a | 10.1 ± 2.9a | 20.2 ± 3.3b | 266.6 ± 32.7d |
| Control | 9.4 ± 1.4a | 9.0 ± 2.2a | 18.4 ± 2.9a | 239.4 ± 32.5c |
| SH 0.6 | 10.2 ± 1.7a | 10.4 ± 2.8b | 20.6 ± 3.8b | 232.5 ± 12.3b |
| SH 1.2 | 9.9 ± 1.5a | 8.9 ± 3.6a | 19.8 ± 4.4a | 257.8 ± 43.9d |
| SH 2.5 | 10.1 ± 1.3a | 10.0 ± 2.9a | 20.0 ± 3.6a | 295.3 ± 8.6e |
| SH 5.0 | 9.5 ± 1.7a | 9.2 ± 2.3a | 18.7 ± 3.2a | 230.0 ± 5.9b |
| SH 10 | 9.6 ± 1.6a | 10.2 ± 2.3a | 19.8 ± 3.1a | 219.9 ± 15.7a |
| SH 20 | 10.6 ± 1.8b | 9.0 ± 2.0a | 19.6 ± 3.0a | 313.7 ± 25.1f |
Different letters (a-f) indicate significant differences between the concentrations from each treatment of crude fucoidan EA or SH according to the Least Significant Difference (LSD) mean comparison test (p ≤ 0.05). Values represent the mean of n = 30 plants; ± standard errors.
The majority of the doses of alginate did not show beneficial effect in growth compared to those of the control plants. EA-alginate at 1.2 mg mL‒1 showed significative effect (p ≤ 0.05) on root length and total length (10.1 cm and 20.6 cm, respectively) respect to control plants (9.2 cm and 18.9 cm, respectively) of mung bean (Figure 5B and C). SH-alginate did not show differences significative in the shoot, root, and total length with respect to control plants (Figure 5A-C and Table 3).

Figure 5 Effect of alginate from Eisenia arborea (EA) and Sargassum horridum (SH) at different doses on (A) shoot, (B) root, (C) total length and (D) Dry weight of mung bean. Columns denoted by asterisk are significantly different to control. Values represent the mean of n = 30 plants; bars represent standard errors.
Table 3 Mung bean growth using different doses of alginate from Eisenia arborea (EA) and Sargassum horridum (SH).
| Doses (mg mL‒1) | Shoot length (cm) | Root length (cm) | Total length (cm) | Dry weight (mg) |
|---|---|---|---|---|
| Control | 9.7 ± 1.9a | 9.2 ± 2.3a | 18.9 ± 3.0a | 256.4 ± 29.2e |
| EA 0.6 | 10.0 ± 1.7a | 9.7 ± 2.0a | 19.5 ± 3.0b | 209.1 ± 15.0a |
| EA 1.2 | 10.5 ± 1.6a | 10.1 ± 2.1b | 20.6 ± 2.5b | 218.7 ± 13.9b |
| EA 2.5 | 10.3 ± 1.6a | 9.8 ± 2.2a | 20.1 ± 3.2b | 234.5 ± 9.20c |
| EA 5.0 | 9.9 ± 1.7a | 9.3 ± 2.1a | 19.2 ± 2.7a | 250.4 ± 21.6d |
| EA 10 | 9.5 ± 1.3a | 9.0 ± 1.6a | 18.5 ± 2.5a | 216.6 ± 26.6b |
| EA 20 | 9.6 ± 1.1a | 9.6 ± 1.1a | 19.1 ± 2.4a | 242.6 ± 36.5c |
| Control | 9.7 ± 1.9a | 9.2 ± 2.3a | 18.9 ± 3.0a | 256.4 ± 29.2d |
| SH 0.6 | 10.2 ± 1.3a | 9.2 ± 2.2a | 19.4 ± 2.7a | 226.2 ± 17.6b |
| SH 1.2 | 9.7 ± 1.5a | 9.3 ± 2.4a | 19.0 ± 3.1a | 220.5 ± 9.20a |
| SH 2.5 | 9.5 ± 1.8a | 9.1 ± 2.8a | 18.6 ± 3.9a | 229.0 ± 25.9b |
| SH 5.0 | 9.2 ± 2.1a | 9.3 ± 2.9a | 18.6 ± 4.1a | 225.0 ± 27.0b |
| SH 10 | 9.9 ± 1.5a | 9.3 ± 2.5a | 19.2 ± 3.1a | 225.8 ± 16.1b |
| SH 20 | 9.7 ± 1.5a | 9.3 ± 2.7a | 19.0 ± 3.3a | 237.4 ± 32.7c |
Different letters (a-d) indicate significant differences between the concentrations from each treatment of alginate EA or SH according to the Least Significant Difference (LSD) mean comparison test (p ≤ 0.05). Values represent the mean of n = 30 plants; ± standard errors.
The use of alginates had a negative effect on the dry weights of the mung bean plants (Figure 5D and Table 3).
DISCUSSION
The use of seed priming as a plant growth bio-stimulant method has been shown to be suitable for plant development. Various compounds, such as phytohormones, sugars, and micronutrients, have been used as priming substances for seeds (Hasanuzzaman & Fotopoulos, 2019). However, there are no records of the use of algal polysaccharides as seed priming. This study is the first to report the use of fucoidans and alginates as priming compounds for seeds.
This study showed that the crude fucoidans obtained from S. horridum and E. arborea act as bio-stimulants for seed germination and seedling growth.
It is widely approved that the use of polysaccharide-enriched extracts (PPEs) showed contradictory effects on seed germination mainly with a dose dependent response (Hernández-Herrera et al. 2016; Castellanos-Barriga et al. 2017). In the present study, the presence of bioactive compounds in PEEs that can stimulate or inhibit seed germination may explain this finding.
In another study, seed treated with alginate and ulvan-derived oligosaccharides resulted from Ulva lactuca Linnaeus and Padina gymnospora PEE-treatments was proposed as a potential mechanism of increased germination percentage under in vitro conditions (Hernández-Herrera et al. 2016). Such oligosaccharide can increase activities of several germination enzymes and accelerate seed metabolic activity (Hu et al. 2004). Water uptake by seeds treated with PEEs was more efficient and can also explain the increased germination percentage over the untreated seeds mainly in the first 3 days.
The use of S. horridum fucoidan resulted in greater benefits for root and overall growth at a concentration of 0.6 mg mL‒1, while higher dry weight was obtained with a concentration of 20 mg mL‒1. This result suggests that the use of crude fucoidan as a biostimulant is a viable option to improve plant growth. However, the alginates in this study did not show bio-stimulant activity on mung bean growth.
The variation in bio-stimulant activity reflected in growth depended on the polysaccharide concentration. These effects have also been demonstrated in tomato plants with the use of polysaccharide-enriched extracts (PPEs) from Ulva lactuca and Padina gymnospora in neutral and alkaline conditions (Hernández-Herrera et al., 2016).
Recently Zou et al. (2021) reported the use of fucoidans from the brown alga Macrocystis pyrifera as growth promoter for wheat plants. Therefore, this is the first study that has explored the use of crude fucoidans with this purpose from Eisenia arborea and Sargassum horridum. The results of this work have shown that fucoidans have the potential growth stimulation activity.
The mung bean seeds treated with polysaccharides were able to maintain their humidity for a longer period compared to control plants. In addition, when the mung bean root emerges, it maintains the growth and development availability, because of the polysaccharide effect. In the case of the mung bean plants treated with SH-fucoidan, shoot length showed the greatest growth with the maximum fucoidan concentration (20 mg mL‒1). However, mung bean root length and total plant length showed maximum values with the lower fucoidan concentration of 0.6 mg mL‒1. Whereas the greatest growth stimulation activity of EA-fucoidan was observed in both shoot length and total plant length, at the concentration of 2.5 and 10 mg mL‒1, respectively. However, these values were lower compared to those of S. horridum. Although both extracts belong to the same family of fucoidans, variations in chemical structure or molecular weight can result in different activities (Lim et al., 2014; Zhao et al., 2018; Fitton et al., 2019; Koh et al., 2019).
The plant growth stimulation activity observed with fucoidans in this study may be because fucoidans are sulphated polysaccharides and alginates are not. The ability of fucoidans to act as plant growth promoters can be explained because the sulphate groups (Rachidi et al., 2020). The sulphate groups present in PPEs have shown positive correlations with shoot and root length, dry weight, and chlorophyll content in tomato plants (Mzibra et al., 2018).
The low growth stimulation activity of alginates may be the result of their chemical structure or that activity occurs only when their oligosaccharides are available. It has been shown that hydrolysed alginate (mono and oligosaccharides) increases bio-stimulant activity, which is reflected in plant growth (Iwasaki & Matsubara, 2000; Idrees et al., 2011; González et al., 2013). The sodium alginate oligosaccharides from Lessonia trabeculata Villouta & Santelices and L. vadosa Searles (= L. flavicans Bory), have been found to result in length and weight increases in tobacco plants (Laporte et al., 2007), and the same has been found with alginates from L. vadose (= L. flavicans), in wheat plants (Chandía et al., 2004).
In a previous study, it was observed that the application of S. horridum liquid extract on mung bean seeds had a negative effect (Di Filippo-Herrera et al., 2019). However, in this study it was found that applying only fucoidan has a positive effect on the growth of both seeds and seedlings. Moreover, both the liquid extract from E. arborea (Di Filippo-Herrera et al., 2019) and the fucoidan obtained in this study showed higher activities at a higher concentration, especially regarding promoting root length (21 and 10.1%) and total length (27 and 20.2%), respectively.
The best result of the polysaccharide pre-treatment on mung bean seeds was observed in dry weight. Similar effects have been previously reported with the lengths of tomato plants treated with PPEs from the brown seaweed Padina gymnospora (Hernández-Herrera et al., 2016) and with sodium alginate-treated lettuce (Lactuca sativa) (Iwasaki and Matsubara, 2000). Mzibra et al. (2018) showed that the PPEs from the brown seaweeds Fucus spiralis and Bifurcaria bifurcata resulted in significant differences in tomato plant length (growth of 338 and 337 mm, respectively) compared to the growth of control plants. Similar results were obtained regarding dry root weight with the PPE from F. spiralis (0.26 g) and in dry shoot weight with the PPE from B. bifurcate (0.81 g), both of which were significantly different from those of the control.











 nueva página del texto (beta)
nueva página del texto (beta)



