INTRODUCTION
The high plant feedstuff inclusion levels influence the existence of mycotoxicosis in aquaculture production in finished feeds (Gonçalves et al., 2020). Aspergillus spp., Penicillium spp., and Fusarium spp. are the main fungal species responsible for most mycotoxins present in meals (Kabak, 2009; Freire & Da-Rocha, 2017). Recent reports of a mycotoxin survey revealed the presence of aflatoxin (AF), zearalenone, T-2 toxin, and deoxynivalenol in numerous feedstuff samples (21,709) collected from 79 countries, from which it was concluded that 6.5 samples out of 10 had at least one of the mentioned mycotoxins above the threshold levels, and 87% of the test samples contained ten or more mycotoxins and metabolites, being AF the most found (Biomin, 2021a). The incidence of mycotoxins is said to be influenced by harvest and storage conditions. For instance, hot and humid environments are the main factors that promote fungal contamination and toxin production (Saad, 2016). Particularly for Aspergillus species, temperature between 25 and 35ºC are known to promote AF development (Daou et al., 2021). These conditions can occur in most aquaculture facilities. Several studies have demonstrated up to 24 different types of mycotoxins (including metabolites) in raw ingredients and complete fish feeds (Koletsi et al., 2021). Deoxynivalenol, AF, zearalenone, ochratoxin, fumonisin B1, fumonisin B2, fusaric acid, ergotamine, and deoxynivalenol-3-glucoside were the most predominant mycotoxins (Gonçalves et al., 2017; Koletsi et al., 2021).
The effects of feeding mycotoxin contaminated diets to fish and shrimp has resulted in significant reduction in feed consumption and growth rate, immune suppression, liver lesions, alterations of phase I xenobiotic biotransformation enzymes such as cytochrome P450 (CYP450) and alkaline phosphatase (ALP), phase II enzymes such as gluthatione S-tranferase (GST) and mortality (Santacroce et al., 2008; Ghaednia et al., 2013; Mahfouz & Sherif, 2015; Pérez-Acosta et al., 2016; Zeng et al., 2016; Tapia-Salazar et al., 2017; Yu et al., 2018). From all mycotoxins, aflatoxins (AFs) are particularly important due to their toxicity and carcinogenic effects (IARC, 2012). Aflatoxin B1 is considered one of the most toxic compounds for terrestrial and aquatic species (Mohamed et al., 2017). Different strategies had been developed to reduce the toxic effect of mycotoxin consumption, such as physical decontamination, chemical decontamination, and biological decontamination (Daou et al., 2021). Earlier studies showed that the use of AF binders such as mixtures of aluminosilicate minerals and Saccharomyces cerevisiae (S. cerevisiae) yeast cell walls; mixtures of glucomannans from cell walls of S. cerevisiae with Chlorella vulgaris cell walls; as well as mixtures of bentonite (a montmorillonite rich mineral), Trichosporon mycotoxinivorans yeast and algae extracts included at levels that the manufacturing industry recommends can improve but not revert toxic effects of diets contaminated with 75 µg/kg AFs on shrimp (Tapia-Salazar et al., 2017). Tapia-Salazar et al. (2017) observed that feeding diets containing AF + 2.5 g/kg MyP resulted in a similar feed intake to the CD diet. The inclusion levels for MyP used for these authors were selected from terrestrial studies due to the lack of information on aquatic organisms. Therefore, more information related to the effectivity of Mycofix Plus® (MyP), to reduce aflatoxicosis at lower inclusion levels in shrimp is required. The objective of the present study was to evaluate the protective effects of different levels of MyP on white shrimp Litopenaeus vannamei (L. vannamei) fed 200 μg/kg AF-contaminated diet in terms of growth rate, survival, feed conversion ratio and the enzymatic activity of ALP and GST after 42 feeding days.
MATERIALS AND METHODS
Preparation of contaminated corn: The AF contamination of corn was performed at the Nutek S.A. de C.V. company facilities in Tehuacan, Puebla, Mexico. The contamination protocol followed methods previously described by Tapia-Salazar et al. (2012). Spores from Aspergillus parasiticus were inoculated onto white corn grains inside an Erlenmeyer flask and then placed in an incubator at 25°C for 7 days. Contaminated corn was then sterilized for 30 min at 120°C, left to dry for 5 days in a biosafety recirculation hood, then milled with a grinder and passed through a steel sieve with an opening size of 850 µm (ASTM mesh #20). Total AFs were measured with high-performance liquid chromatography (HPLC) following the official AOAC method 994.08 (AOAC, 2007).
Preparation of experimental diets: To assure optimal growth of juvenile shrimp, a non-contaminated control diet (NCD) containing 40% crude protein and 7% lipid content was prepared with wheat flour (43%), soybean meal (8%) and shrimp meal (4%) as main ingredients. The AF contaminated diet (ACD) was prepared by adding 10.9% of contaminated cornmeal in substitution for the wheat flour. The AF content in ACD (200 μg/kg) was based on a previous study where a significant reduction in shrimp growth, feed intake, and survival was observed (García-Pérez et al., 2020). Three more experimental diets were prepared with ACD complemented with 1, 1.5 or 2 g of MyP per kg of diet. Briefly, the ingredients for diet preparation were ground in a CyclotecTM 1093 sample mill (Foss-Tecator, Denmark) to obtain an average particle size of 500 μm, after milling, the ingredients were mixed with soy lecithin and fish oil for 10 min in a Kitchen Aid mixer and then warm water (30%) was added to be mixed for 15 more min. Finally, the dough was processed through a grinder (fitted with a metal screen with 1.6 mm hole diameter) at a 40 min/kg passage rate at 75ºC. The pellets were then dried in a convection oven at 100ºC for 8 min and were left to cool overnight before placing on Ziploc bags. The chemical composition of experimental diets (Table 1) was calculated using previously reported methods (Cruz-Suárez et al., 2009). Total AFs in the diet were measured by a fluorometer method using AflaTest® immunoaffinity columns (VICAM, Milford, MA). Columns can detect the four most common AFs expected to be found in feed, such as AFB1, AFB2, AFG1, and AFG2. Proximate analysis on diets was done to determine dry matter (%) and water absorption (%) following methods previously reported (García-Pérez et al., 2013).
Table 1 Composition and proximate analysis of experimental diets for juvenile white shrimp
| NCD | ACD | ACD+ 1 MyP | ACD+ 1.5 MyP | ACD+ 2 MyP | |
| Formula (g/kg) | |||||
| Wheat meal | 431.26 | 431.47 | 435.18 | 434.59 | 434.1 |
| Fish meal | 380.18 | 379.97 | 387.87 | 387.99 | 388.06 |
| Soybean meal | 80 | 80 | 67.4 | 67.45 | 67.34 |
| Constant ingredients† | 97.6 | 97.6 | 97.6 | 97.6 | 97.6 |
| Non-contaminated corn | 10.953 | --- | --- | --- | --- |
| Contaminated corn | --- | 10.953 | 10.953 | 10.953 | 10.953 |
| MyP | --- | --- | 1 | 1.5 | 2 |
| Total | 1000.00 | 1000.00 | 1000.00 | 1000.00 | 1000.00 |
| Chemical composition (% dry matter) | |||||
| Moisture | |||||
| Protein | 42.6 ± 0.3 | 42.5 ± 0.09 | 42.1 ± 0.2 | 42.2 ± 0.2 | 42.2 ± 0.3 |
| Crude lipids | 7.3 ± 0.3 | 7.3 ± 0.4 | 7.2 ± 0.3 | 7.4 ± 0.3 | 7.3 ± 0.4 |
| Fiber | 2.9 ± 0.02 | 2.9 ± 0.1 | 2.6 ± 0.03 | 2.5 ± 0.09 | 2.8 ± 0.2 |
| Ash | 10.4 ± 0.01 | 10.6 ± 0.1 | 10.4 ± 0.02 | 10.5 ± 0.1 | 10.9 ± 0.02 |
†Constant ingredients (g/kg): shrimp meal 40, fish oil 20, soy lecithin 20, alginic acid 10, vitamin mixture 3.5, mineral mixture 2.5, antioxidant 0.5, mold inhibitor 0.5, cholesterol 0.2, vitamin C 0.2 and vitamin E 0.2
Vitamin mixture composition: retinol, 4000 IU/g; thiamin, 24 g/kg; riboflavin, 16 g/kg; DL Ca pantothenate, 30 g/kg; pyridoxine, 30 g/kg; cyanocobalamin, 80 mg/kg; ascorbic acid, 60 g/kg; menadione, 16 g/kg; cholecalciferol, 3200 IU/g; tocopherol, 60 g/kg; biotin, 400 mg/kg; niacin, 20 mg/kg; folic acid, 4 g/kg.
Mineral mixture composition: Co, 2 g/kg; Mn, 16 g/kg; Zn, 40 g/kg; Cu, 20 g/kg; Fe, 1 mg/kg; Se, 100 mg/kg; I, 2 g/kg.
Animal study conditions: Juvenile shrimp of L. vannamei species were maintained in a closed recirculation system containing artificial seawater (Fritz®, Dallas, TX, USA) using a 350 mL/min flow-through rate. Each tank was equipped with an air-water lift system for internal recirculation. All tanks were interconnected to achieve the same conditions simultaneously. The study did not require ethical approval to develop research with invertebrates as it is unnecessary by the Mexican regulations. However, studies were developed adhering to maintenance and euthanasia protocols reported for decapods (Approved protocols for decapods, cephalopods, and fish, 2018; Leary et al., 2020). Water temperature and salinity were measured daily while pH, ammonium, nitrates, and nitrites, were measured weekly.
Shrimp source and feeding protocol: For this study, juvenile shrimp were obtained from Municipio de Rosario, Sinaloa, Mexico, through a donation by a local farm. Upon arrival, shrimp were acclimated for 4 days in 500 L tanks. After acclimation, shrimp were individually weighed (75 mg average initial weigh) and allocated to 15 tanks (12 shrimp per tank). Five study groups were formed as follows: NCD, ACD, ACD + 1, 1.5, or 2 g of MyP per kg of diet, and each group had three replicates. Replacement animals were kept in a tank and when necessary, dead shrimp were replaced over the first three days after distributing the animals in each study group. Shrimp were kept on 12/12 light/dark cycle conditions for 42 feeding days. The initial feeding ratio was based on a 20% total biomass found in each tank. Feeding protocol and calculation of feeding rate were based on previous research (Tapia-Salazar et al., 2012). Shrimp were fed 3 times a day (8:00 am, 12:00 pm and 5:00 pm); siphoned leftover feed from the tank before the new fresh feed was provided. Pelleted feed was broken down into small crumbs to ensure meal was available to all shrimp.
Growth parameters: During the experiment, shrimp from each experimental group were individually weighed at 0, 14, 28, and 42 days using a digital scale. The amount of feed provided per tank was adjusted every two weeks after shrimp weight. Before weighing, excess water in shrimp was removed with a cotton cloth. Survival and feed consumption were recorded daily; appropriate feed adjustments were done considering the remaining shrimp and leftover feed in each tank, every day. Growth rate, feed consumption, feed conversion ratio, and survival rate were calculated by using formulas described by García-Pérez et al. (2013). A sample of shrimp (10 g) from the replacement tank was taken at time 0 and at the end of the experiment to measure water and nitrogen content in shrimp tissue. Shrimp samples for nitrogen content were freeze-dried and then ground in a mill. Calculation of nitrogen retention efficiency was done according to the following formula:
Were:
AFW |
Average final weight |
FCP |
Final crude protein in carcass |
AIW |
Average initial weight |
ICPC |
Initial crude protein in carcass |
CCP |
Consumed crude protein |
Enzymatic activity biomarkers: At the end of the experiment, hepatopancreas samples of three shrimp from each tank were taken to measure ALP and GST activities. Hepatopancreas samples were homogenized in double distilled water at 4 ºC in a 1:10 proportion (sample weight:water, m/v) with a mortar and pestle for 4 minutes. Then homogenized samples were centrifuged at 2000 g for 15 minutes at 4 ºC, and the supernatant was aliquoted into 0.1 mL Eppendorf tubes and stored at -70 ºC until use. Protein content in tissue extracts was quantified using Bradford method and bovine serum albumin (BSA) as a calibration curve (Bradford, 1976). ALP activity was determined by using p-nitrophenyl phosphate (substrate). The reaction was performed using 200 µL of diethanolamine buffer (1.0 M) with 50 mM MgCl2 (pH 9.8), then 10 µL of the enzymatic extract and 10 µL of the substrate were added at a final concentration of 0.4 mM. Absorbance was immediately registered at 405 nm in 120-second intervals for up to 10 minutes in an EPOCH microplate reader (Biotek, Vermont, USA). For each sample, three analytical replications were conducted. The sample was replaced with a buffer in control wells. The linearity of the reaction was verified, and the enzymatic activity was expressed as μmol/min/mg protein using p-nitrophenol molar extinction coefficient of 18.5 mM/cm (Mazorra et al., 2002). GST activity was analyzed using the Habig et al., (1974) method adapted to microplates. A volume of 300 μL of a substrate mixture containing reduced L-glutathione (200 mM) and 1-chloro-2, 4-dinitrobenzene (CDNB; 100 mM) in Dulbecco’s phosphate-buffered saline (2.7 mM KCl, 1.5 mM KH2PO4, 136.9 mM NaCl and 8.9 mM Na2HPO4•7H2O, pH 7.2) along with 10 μL of hepatopancreas extract were added into each well. Absorbance was immediately read at 340 nm every minute for 10 minutes. GST activity was expressed as μmol/min/mg protein, using a molar extinction coefficient of 9.6 mM/cm for CDNB (Brodeur et al., 2011).
Statistical analysis: Statistical analyses were done using SSPS software (version 16.0, SPSS Inc., Chicago, Illinois). Average body weight per tank was used to calculate the growth rate and feed conversion ratio. Normality of data was not verified as it is not required for sample size ≥ 25 (Van den Berg, 2022). Homoscedasticity (homogeneity of variances) was verified with Levene´s test. The test variables (growth parameters and enzymatic activity) were analyzed with a one-way ANOVA test and then followed by Tukey´s multiple comparisons tests to detect significant differences among experimental groups (p <0.05).
RESULTS
Experimental diets: Chemical composition of the experimental diets was similar among treatments (Table 1). The concentration of total AFs in ACD was 200 µg/kg while NCD did not show detectable levels of AFs (Limit of detection=1 ng AF/kg diet). Values of dry matter loss and water absorption capacity of diets are presented in Table 2. One-way ANOVA showed that MyP addition at 1.5 and 2 g/kg had a higher reduction of water absorption (p = 0.001) while NCD had a significantly lower dry matter loss when compared to ACD and ACD + 1.5 diets (p < 0.001) but not significantly different from ACD + 1 MyP or ACD + 2 MyP.
Table 2 Dry matter loss (DML) percentage and water absorption capacity (WA) of experimental diets.
| Experimental diet | DML (%) | WA (%) |
|---|---|---|
| NCD | 16.1 ± 0.01a | 182 ± 3.6c |
| ACD | 18.1 ± 0.7bc | 164 ± 20bc |
| ACD+1 MyP | 17.3 ± 0.1ab | 141 ± 6b |
| ACD+1.5 MyP | 19.3 ± 0.3c | 104 ± 4a |
| ACD+2 MyP | 16.9 ± 1.1ab | 109 ± 10a |
| SEM | 0.32 | 8.45 |
| Sig. | 0.001 | 0.001 |
Data presented are the means ± standard deviations. Different letters in the same column indicate significant differences among groups with Tukey mean comparisons (p < 0.05).
Animal study conditions and growth parameters: Water condition parameters (mean ± standard deviation) were maintained during the study with the following values recorded for salinity 35 ± 3 g/L, temperature 30 ± 2 ºC, pH 8.1 ± 0.1, ammonium 0 mg/L, nitrites 0.2 mg/L, and nitrates 40 ± 15 mg/L. Shrimp final growth parameters such as weight, feed intake, growth rate, feed conversion ratio, survival (%) and nitrogen retention efficiency (%) are presented in Table 3. When compared to NCD, one-way ANOVA showed that shrimp consuming ACD had a significant reduction in growth, feed intake and nitrogen retention efficiency (p < 0.05). Interestingly, shrimp from all MyP groups had mean weight, growth rate and nitrogen retention efficiency similar to animals from NCD. Particularly, animals feed with ACD + 2 MyP showed no differences (p < 0.05) in mean weights, feed intake, growth rate, or nitrogen retention efficiency when compared to animals from NCD group. However, animals with the low and medium dose of MyP (i.e., 1 and 1.5 g of MyP) did not reach significance to be different from animals on ACD. No significant differences were observed for feed conversion ratio or survival. An apparent improvement in feed intake was noted due to MyP addition to ACD, however, only the 2 g/ kg inclusion of MyP showed a significant difference among study groups, resulting on a higher average feed intake of 3.8 g compared to the 2.9 g observed in ACD group. Regarding the growth rate, shrimp that consumed ACD + 2 MyP had higher growth compared to the shrimp fed with ACD (2980% vs 2185% respectively), and it was similar to the growth rate of shrimp consuming NCD (2964%). Nitrogen retention efficiency was lower for the shrimp consuming ACD (21.7%) than that for shrimp of NCD and ACD+2 MyP groups (35.6% and 35.01% respectively), animals from these treatment groups had the highest nitrogen retention values (p=0.007).
Table 3 Growth parameters of white shrimp fed non-contaminated diet (NCD) or aflatoxin-contaminated diet (ACD) supplemented with MyP
| Experimental diet | Mean weight (g) | Feed intake (g/shrimp) | Growth rate (%) | Feed conversion ratio | Survival (%) | Nitrogen retention efficiency (%) |
|---|---|---|---|---|---|---|
| NCD | 2.34 ± 0.17b | 4.07 ± 0.2c | 2964 ± 234b | 2.59 ± 0.1 | 97.2 ± 4.8 | 35.6 ± 3.6b |
| ACD | 1.73 ± 0.1a | 2.99 ± 0.1a | 2185 ± 137a | 3.07 ± 0.1 | 97.2 ± 4.8 | 21.7 ± 2.3a |
| ACD+1 MyP | 1.92 ± 0.2ab | 3.3 ± 0.2ab | 2432 ± 274ab | 2.88 ± 0.3 | 100 ± 0 | 29.4 ± 3.3ab |
| ACD+1.5 MyP | 2.02 ± 0.3ab | 3.24 ± 0.2ab | 2500 ± 489ab | 2.76 ± 0.6 | 94.4 ± 4.9 | 29.5 ± 2.9ab |
| ACD+2 MyP | 2.33 ± 0.8b | 3.84 ± 0.07bc | 2980 ± 137b | 2.3 ± 0.1 | 91.6 ± 8.4 | 35.01 ± 1.6b |
| SEM | 0.078 | 0.211 | 104 | 0.101 | 1.37 | 1.62 |
| Sig. | 0.023 | 0.008 | 0.025 | 0.123 | 0.415 | 0.007 |
Data presented are the means ± standard deviations. Different letters in the same column indicate significant differences with Tukey mean comparisons (p < 0.05)
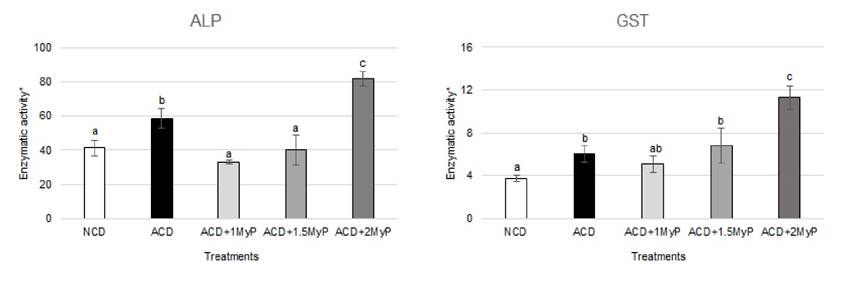
Enzymatic activity: Shrimp fed with ACD had a higher ALP and GST activity than organisms fed with NCD (Figure 1). Regarding ALP, the shrimp from ACD + 1 MyP and ACD + 1.5 MyP groups had the lowest enzymatic activity values and were similar to the shrimp from the NCD group. On the contrary, the shrimp from the ACD + 2 MyP group showed the highest enzyme activity value, and this difference was significant when compared to all other treatment groups (p < 0.05). GST activity did not result in significant differences among the ACD + 1 MyP and ACD + 1.5 MyP groups, however, shrimp belonging to the ACD + 2 MyP group had the highest enzyme activity among all treatment groups, including the ACD group (p < 0.05). Overall, a trend of increased activity was observed for both enzymes as higher concentrations of MyP were present in the diets.
DISCUSSION
The current study showed that ACD consumption by shrimp for a 42-day period resulted in significant reductions in growth parameters such as mean weight, feed intake, growth rate, and nitrogen retention efficiency. These results are consistent with those published in previous studies where consumption of AFs resulted in evident adverse effects in shrimp of the same species and age, and under similar experimental conditions (Tapia-Salazar et al., 2012; García-Perez et al., 2020), and in shrimp of similar species such as Penaeus monodon (Bautista et al., 1994; Gopinath & Paul-Raj, 2009). The recurring dietary exposure to AFs exerts a substantial impact on shrimp farming, causing subclinical symptoms that subsequently result in impaired health status, followed by decreased production efficiency (Wang et al., 2018). In livestock production, the addition of mycotoxin binders or AFs adsorbents to the diets is an economical strategy to prevent the negative effects caused by the presence of these toxins in feed (Farooqui et al., 2019). According to Neeratanaphan & Tengjaroenkul (2018), the use of Thai bentonite has been shown to improve weight gain, survival rate, red blood cell counts and alanine aminotransferase enzyme in white shrimp feed diet supplemented with 0.5% bentonite when compared to those fed toxin contaminated diet only (150 ppb AFB1). Most mineral-based binders claim to be effective for AF sorption and this has been supported by in vitro and in vivo studies where montmorillonites (Ramos & Hernández, 1996), and zeolites (Piva et al., 1995) have shown a high in vitro binding affinity for AFs, and hydrated sodium calcium aluminosilicates (HSCAS) (Döll et al., 2005) and bentonites (Schell et al., 1993) have proven to reduce the harmful effects of this toxin on performance parameters in other species such as pigs. Some mycotoxin binders are also formulated with a variety of other ingredients including antioxidants, probiotics, yeast, or plant materials that may improve their effectiveness (Tapia-Salazar et al., 2017). That is the case of MyP which is also described as a mycotoxin deactivator (Hanif et al., 2008). Since the adsorption of AF to mineral binders is essentially a surface phenomenon, its effectiveness depends on several physical characteristics such as pore size and distribution, total charge, and charge distribution (Di-Gregorio et al., 2014). In the present study, shrimp that were fed diets supplemented with MyP had increased mean weight, feed intake, growth rate and nitrogen retention efficiency, while the shrimp that consumed the ACD + 2MyP presented significant differences when compared with the shrimp fed with ACD. Regarding cost-benefits, by using our FCR data, an approximate price of 32 Mexican pesos (Mxp) for a regular 1 kg of shrimp diet, and 0.22 Mxp as the approximate cost of 1 g of MyP, the cost of production of 1 kg of shrimp with a regular shrimp diet contaminated with AF (200 µg/kg) and complemented with 1, 1.5 or 2 g/kg MyP has an average estimate of 85 Mxp, similar to the cost of production using non-contaminated diet (83 Mxp), and contrarily to what is expected for shrimp only ingesting AF contaminated diet (98.24 Mxp).
The use of mycotoxin binder or deactivator products has been successfully used in terrestrial animals to prevent the effect of consuming AFs (Hussain et al., 2017; Kehinde et al., 2018; Nazarizadeh & Pourreza, 2019; Saleemi et al., 2020), however, in aquaculture, few studies have used mixed-nature adsorbents that contain ingredients that are known to modulate xenobiotic biotransformation enzymes. Agouz & Anwer (2011) found an increase in growth and survival in common carp (Cyrpinus carpio) supplemented with a synthetic probiotic-immunostimulant product (Biogenâ) and a commercial smectite clay composed of an activated, broad-spectrum HSCAS (Myco-Adâ) in a diet contaminated with 22 ppb AF and 15 ppb ochratoxin. Other studies showed that shrimp ingesting a diet with 75 μg AF/kg and supplemented with 2.5 g/kg MyP, improved the growth rate, although not significantly (Tapia-Salazar et al., 2017). The reasons for the differences found between the Tapia-Salazar et al. (2017) study and our current study are likely related to the different AFs contamination levels (75 µg/kg AFs vs 200 µg/kg), dietary inclusion amounts of MyP product (2.5 g/kg diet vs 1, 1.5 and 2 g/kg diet), as well as the average initial weights of the organisms used (210 mg vs 76 mg).
Regarding enzymatic activity, the present study found an increase in ALP and GST activities in shrimp fed with ACD when compared to those fed with NCD. The increase in enzymatic activity due to the consumption of AF is consistent with previous results observed in the aquatic organisms (Mahfouz & Sherif, 2015; Manal, 2016). ALP is a hepatopancreatic enzyme involved in detoxification, elevated ALP activity is found in the hepatopancreas when this organ needs to metabolize large amounts of xenobiotics (Boonyaratpalin et al., 2001). Previous works (García-Pérez et al., 2020) showed that ALP activity can increase in shrimp consuming aflatoxins (200 ppb) when compared to animals consuming non-contaminated diet (58.4 µmol/min/mg protein vs 41.3 µmol/min/mg protein, respectively). Contrarily, some studies show a reduction in hepatopancreas ALP activity in enzyme extracts incubated with AFB1 (Perez-Acosta et al., 2016), however, in vitro data may not reflect what occurs in vivo. Increased GST activity has also been shown in shrimp exposed to AFB1 at levels as low as 15 ppb (Zhao et al., 2017). Clearly, increased activity of both enzymes can be beneficial to shrimp that are exposed to AFB1-contaminated diets.
Interestingly, in the current study, shrimp from ACD + 1 MyP and ACD + MyP 1.5 did not show significant differences in ALP activity when compared to animals from NCD group. Thus, highlighting the beneficial effects of dietary inclusion of MyP at either 1 or 1.5 g/kg inclusion. MyP is a clay-based feed additive frequently used as an AF adsorbent. The mineral components belong to the aluminosilicate group, specifically, the bentonite type (rich in montmorillonite), according to the manufacturer. Bentonites have a high capacity for AF adsorption (Thieu & Pettersson, 2008) and the mechanisms of toxin adsorption have been described as chemisorption on mineral surfaces (Grant & Phillips, 1998) or physisorption with the interlayer cations (Deng et al., 2010). Upon binding, a complex toxin-mineral is formed thus reducing AF bioavailability from the gastrointestinal tract (Ramos & Hernández, 1997), which may prevent hepatopancreas damage and the consequent changes in ALP and GST enzyme activity.
Among treatments, the shrimp belonging to the ACD + 2 MyP group had the highest values of both enzymes, even higher than shrimp fed ACD. It is known that MyP contains a proprietary blend of plant and algae extracts said to support liver function (Biomin, 2021b). For instance, Abdel-Rahim et al., (2021) reported that when feeding juvenile Litopenaeus vannamei with 500 mg/kg of Sargassum polycystum algae supplemented feed, the ALP activity showed a higher mean of 8.80 U/L compared to the 6.33 U/L mean registered for control animals (no-algae supplemented feed). The source of algae in MyP is not known (undisclosed for patent protection) but seaweed and macroalgae are a rich source of bioactive compounds (Thanigaivel et al., 2014), and some have hepatoprotective activity (Schleder et al., 2018). Hence, we proposed that the inclusion of MyP at 1 and 1.5 g/kg inclusions were able to maintain ALP and GST enzymatic activities similar to those values observed for control animals. However, at a higher inclusion of MyP (2 g / kg), other bioactive compounds present in this product may stimulate hepatopancreas xenobiotic metabolic functions resulting on increased ALP and GST enzyme activities.
CONCLUSION
Feeding MyP to juvenile L. vannamei shrimp ingesting 200 µg/kg of AF was able to reduce the negative effects exerted by the toxin on growth parameters and on enzymatic activities. Overall health and performance of all shrimp ingesting MyP were similar that animals fed NCD. This was especially observed in animals with the high dose of MyP (2 g/kg). Additionally, the high dose of MyP (2 g/kg) resulted in enzymatic ALP and GST activities higher than all other groups, including control animals and animals fed ACD suggesting a further hepatopancreas xenobiotic-metabolizing enzymes stimulation caused by other bioactive compounds in MyP. Further studies can be directed to identify the specific bioactive compounds on MyP and to delineate if there is an additive or synergistic effect on MyP ingredients and AF on ALP and GST enzymes.











 nueva página del texto (beta)
nueva página del texto (beta)