INTRODUCTION
Chydoridae is the most diverse family within cladocerans, but information regarding their culture and biology is still scarce in comparison to other families like Daphniidae and Moinidae. In natural environments, fish larvae can feed on little size species like chydorids, which are preferably consumed over other large cladocerans, likely because of their ease to capture (Nunn et al., 2012). Chydorids, which inhabit littoral zones of water bodies, are generally associated to aquatic plants, periphyton, or sediment (Dole-Olivier et al., 2000; Masclaux et al., 2012). Thus, different media have been developed to culture chydorids in laboratory conditions, feeding these organisms on algae, bacteria, detritus, yeast, or combinations of these sources of organic matter (Martínez-Jerónimo & Gomez-Díaz, 2011).
Castilho et al. (2015) stated that the study of the life cycle of cladocerans offers a better understanding of their biology, and at higher hierarchy level provide information about population dynamics, their interactions with the surroundings, and their role within food webs and secondary production.
Alona guttata (Sars, 1862) is a cosmopolitan species, with records in several places around the world (Sinev & Siva-Briano, 2012; Sousa et al., 2014). Cortez-Silva et al. (2022) described the life history of A. guttata fed on Raphidocelis subcapitata (Korshikov) Nygaard, Komárek, J.Kristiansen & O.M.Skulberg 1987, and the strategies that this species might follow to rapidly colonize temporal and transitory ponds. Alvarado-Suárez (2017) implemented this chydorid to nourish the goodeid fish Poeciliopsis infans (Woolman, 1894) and concluded that A. guttata is of high nutritional value but their usage is limited due to poor biomass production. Therefore, this study was aimed to assess the effect of the food source, algal concentration, and temperature on the demography of A. guttata through life table analysis and find the best culture conditions in terms of the population growth rates.
MATERIAL AND METHODS
Maintenance of chydorids. The strain of A. guttata has been maintained in the Laboratory of Aquatic Toxicology of the Universidad Autónoma de Aguascalientes (UAA) and was cultured with the following conditions: moderately hard reconstituted water (MHRW) (USEPA, 2002), photoperiod 16h light and 8h dark, fed once a day ad libitum with either the green algae Chlorella vulgaris (Beijerinck, 1890) or Nannochloropsis oculata (Hibberd, 1981), and temperature at either 20°C or 25°C ± 2ºC. Freshwater media was supplemented with 660 mg/L of artificial substrate, which consisted of 70% silica sand, 25% kaolin, and 5% dried and ground cattle manure. The complete mixture of the substrate was sterilized by autoclave at 15 psi for 20 min (Martínez-Jerónimo & Gómez-Díaz, 2011).
Algal biomass as food source for chydorids. Chlorella vulgaris and N. oculata were grown in Bold’s Basal medium (Nichols, 1973) under aseptic conditions at 25ºC. Continuous illumination was provided by day-light fluorescent lamps (approximately 5000 lux). Algal biomass was collected during the exponential phase of population growth and separated from the culture medium by centrifugation at 5000 rpm for 5 min. Then, culture media for chydorids were supplemented with either C. vulgaris or N. oculata at low (0.5×106 cells/mL) and high densities (2×106 cells/mL).
Effect of diet and temperature on Alona guttata. Cohorts of five neonates (less than 24-h old) of A. guttata were placed separately in a 24-well polystyrene microplate; then, every well was supplemented with the corresponding algal suspension in MHRW and adjusted to a final volume of 2 mL. Temperature was controlled in bioclimatic chambers at either 20ºC or 25ºC ± 2ºC with a photoperiod of 16 h light and 8 h dark. To avoid desiccation, 200 μL of distilled water were added every other day to each well. Complete renewal of the culture media was performed once a week. Fertility and survival data were daily recorded until all individuals in the cohort had died. The neonates and dead organisms were removed and counted daily. All treatments consisted of six replicas (n = 6).
Life table analysis. Data of survival (lx) and fertility (mx) were used to determine: average lifespan (ALS) (days), maximum longevity (days), life expectancy at birth (LEB) (days), generation time (GT) (days), gross reproductive rate (GRR) (neonates/female), net reproductive rate (NRR) (neonates/female), and intrinsic rate of population increase (r) (1/days) (Pianka, 1978; Krebs, 1985):
Average lifespan
Life expectancy at birth
Gross reproductive rate
Net reproduction rate
Generation time
The intrinsic rate of population increase (IRPI) was computed through iteration with the Euler-Lotka equation:
Statistical Analysis. Results from the life table experiments were analyzed through three-way analysis of variance (ANOVA). Significant differences were established through Bonferroni´s multiple comparison test. Statistical analysis were performed in R-Studio v.1.0.143 and the packages agricolae v.1.2-4 (De Mendiburu, 2016) and ggplot2 v.3.2.1 (Wickham, 2016).
RESULTS
Figure 1 shows the survival curves for A. guttata raised at 20ºC or 25ºC and fed on 0.5×106 or 2×106 cells/mL of either C. vulgaris or N. oculata. The longer survival rates were recorded when organisms were reared at 20°C in medium supplemented with N. oculata. Chydorids fed on N. oculata (2×106 cells/mL) and grown at 20°C delayed until the day 40 of the test to exhibit the first females to die, while at 25°C dead organisms appeared since the day 30. Temperature alone seemed to have no significant effect on the survival of A. guttata, but it was influenced by the interaction of temperature×algal concentration and temperature×algal species.
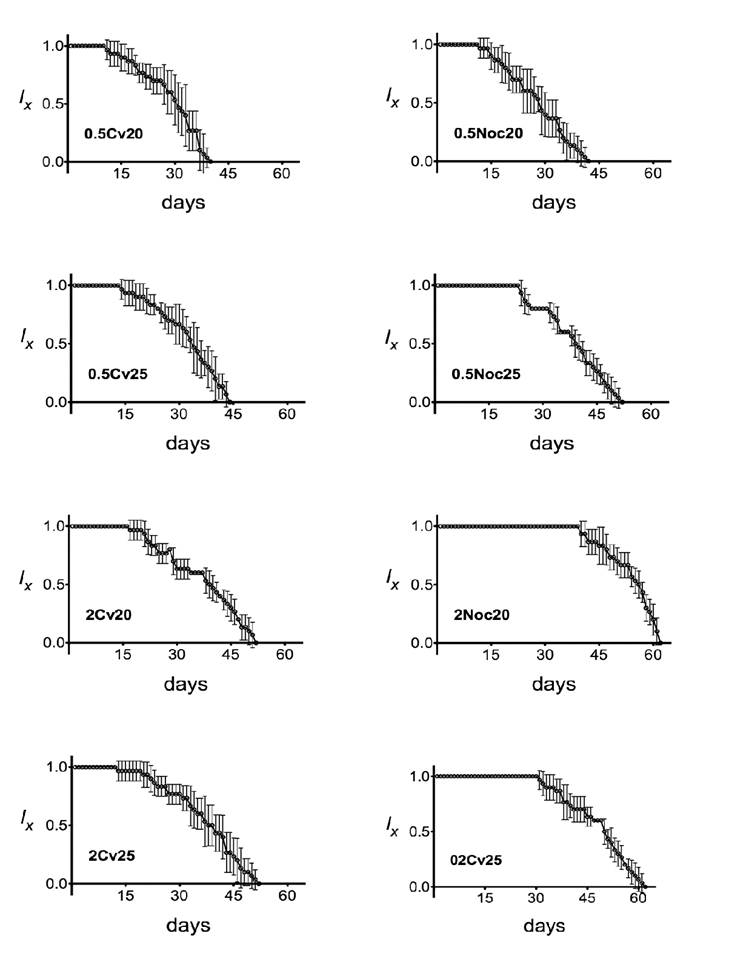
Figure 1 Survival (lx) of Alona guttata (Sars, 1862) fed on Chlorella vulgaris (Beijerinck, 1890) and Nannochloropsis oculata (Hibberd, 1981). Error bars represent the confidence interval (P<0.05). All experiments were carried out with six replicates (n = 6). 0.5 = 0.5×106 cells/mL; 2 = 2×106 cells/mL; Cv = C. vulgaris; Noc = N. oculata; 20 and 25 = temperature in Celsius.
Figure 2 shows the daily fertility of the eight treatments. Alona guttata produced offspring during almost their entire life cycle, although the number of neonates per reproductive episode was in general low. Maximum values of longevity were recorded for every treatment, with the highest values (60.33 ± 0.8164 d) when organisms were fed on N. oculata at 2×106 cells/mL (Fig. 2). The highest longevity was recorded for the group fed on C. vulgaris (49.17 ± 1.8348 d). In general, the concentration of 0.5×106 cells/mL probed to be insufficient to promote longer longevity in Alona. These results were influenced mainly by the algal species and the algal concentration but were not significantly affected by temperature (Table 1).
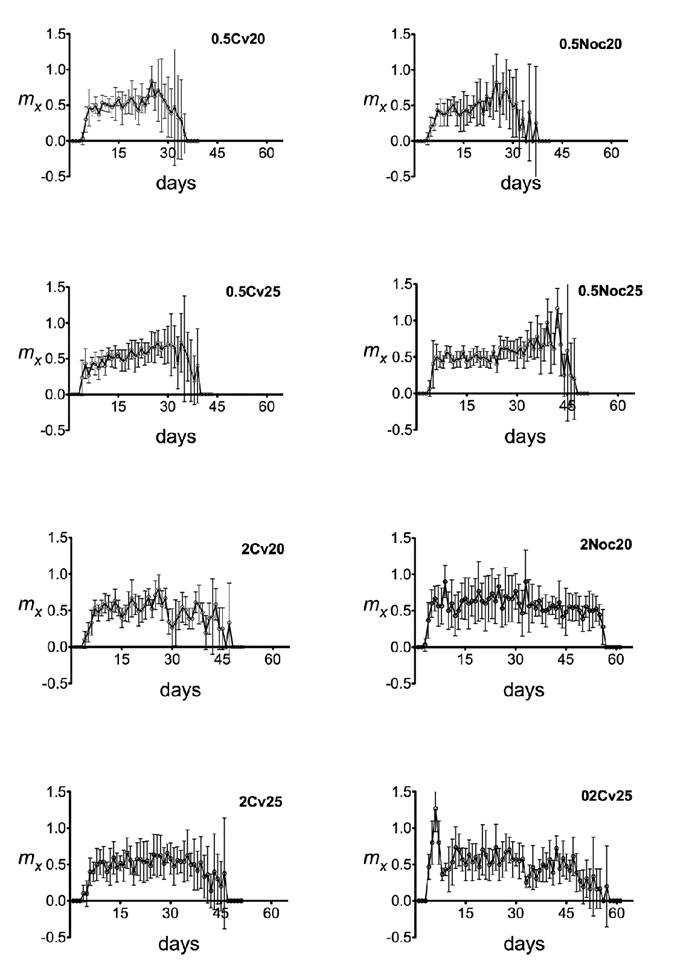
Figure 2 Fertility (mx) of Alona guttata (Sars, 1862) fed on Chlorella vulgaris (Beijerinck, 1890) and Nannochloropsis oculata (Hibberd, 1981). Error bars represent the confidence interval (P<0.05). All experiments were carried out with six replicates (n = 6). 0.5 = 0.5×106 cells/mL; 2 = 2×106 cells/mL; Cv = C. vulgaris; Noc = N. oculata; 20 and 25 = temperature in Celsius.
Table 1 Analysis of variance performed on the demographic responses of Alona guttata fed on Chlorella vulgaris or Nannochloropsis oculata. ALS, average lifespan (days); longevity, maximum longevity recorded by replicate (days); LEB, life expectancy at birth (days); GT, generation time (days); GRR, gross reproductive rate (neonates/female); NRR, net reproductive rate (neonates/female); IRPI, intrinsic rate of population increase, 1/days.
| Factor or interaction | Df | Sum Sq | Mean Sq | F value | Pr(>F) | ||
|---|---|---|---|---|---|---|---|
| ALS | Algal species | 1 | 585.20 | 585.20 | 86.207 | < 0.001 | *** |
| Algae concentration | 1 | 2,192.40 | 2,192.40 | 322.966 | < 0.001 | *** | |
| temperature | 1 | 7.70 | 7.70 | 1.131 | 0.294 | ||
| Algal species × algae concentration | 1 | 588.00 | 588.00 | 86.619 | < 0.001 | *** | |
| Algal species × temperature | 1 | 9.40 | 9.40 | 1.379 | 0.247 | ||
| Algae concentration × temperature | 1 | 162.80 | 162.80 | 23.983 | < 0.001 | *** | |
| Algal species × algae concentration × temperature | 1 | 40.30 | 40.30 | 5.942 | 0.019 | * | |
| Residuals | 40 | 271.50 | 6.80 | ||||
| Longevity | Algal species | 1 | 574.10 | 574.10 | 123.903 | < 0.001 | *** |
| Algae concentration | 1 | 2,352.00 | 2,352.00 | 507.626 | < 0.001 | *** | |
| temperature | 1 | 36.80 | 36.80 | 7.932 | 0.007 | ** | |
| Algal species × algae concentration | 1 | 225.30 | 225.30 | 48.633 | < 0.001 | *** | |
| Algal species × temperature | 1 | 0.70 | 0.70 | 0.162 | 0.689 | ||
| Algae concentration × temperature | 1 | 161.30 | 161.30 | 34.82 | < 0.001 | *** | |
| Algal species × algae concentration × temperature | 1 | 0.30 | 0.30 | 0.072 | 0.79 | ||
| Residuals | 40 | 185.30 | 4.60 | ||||
| LEB | Algal species | 1 | 585.20 | 585.20 | 86.207 | < 0.001 | *** |
| Algae concentration | 1 | 2,192.40 | 2,192.40 | 322.966 | < 0.001 | *** | |
| temperature | 1 | 7.70 | 7.70 | 1.131 | 0.294 | ||
| Algal species × algae concentration | 1 | 588.00 | 588.00 | 86.619 | < 0.001 | *** | |
| Algal species × temperature | 1 | 9.40 | 9.40 | 1.379 | 0.247 | ||
| Algae concentration × temperature | 1 | 162.80 | 162.80 | 23.983 | < 0.001 | *** | |
| Algal species × algae concentration × temperature | 1 | 40.30 | 40.30 | 5.942 | 0.019 | * | |
| Residuals | 40 | 271.50 | 6.80 | ||||
| GT | Algal species | 1 | 38.00 | 38.00 | 23.624 | < 0.001 | *** |
| Algae concentration | 1 | 287.00 | 287.00 | 178.412 | < 0.001 | *** | |
| temperature | 1 | 2.13 | 2.13 | 1.327 | 0.256 | ||
| Algal species × algae concentration | 1 | 22.12 | 22.12 | 13.754 | < 0.001 | *** | |
| Algal species × temperature | 1 | 8.80 | 8.80 | 5.469 | 0.024 | * | |
| Algae concentration × temperature | 1 | 44.93 | 44.93 | 27.929 | < 0.001 | *** | |
| Algal species × algae concentration × temperature | 1 | 15.82 | 15.82 | 9.832 | 0.003 | ** | |
| Residuals | 40 | 64.34 | 1.61 | ||||
| GRR | Algal species | 1 | 0.50 | 0.50 | 0.12 | 0.731 | |
| Algae concentration | 1 | 148.50 | 148.50 | 35.662 | < 0.001 | *** | |
| temperature | 1 | 318.10 | 318.10 | 76.38 | < 0.001 | *** | |
| Algal species × algae concentration | 1 | 1.90 | 1.90 | 0.467 | 0.498 | ||
| Algal species × temperature | 1 | 161.10 | 161.10 | 38.68 | < 0.001 | *** | |
| Algae concentration × temperature | 1 | 11.30 | 11.30 | 2.724 | 0.107 | ||
| Algal species × algae concentration × temperature | 1 | 104.30 | 104.30 | 25.052 | < 0.001 | *** | |
| Residuals | 40 | 166.60 | 4.20 | ||||
| NRR | Algal species | 1 | 254.80 | 254.80 | 180.248 | < 0.001 | *** |
| Algae concentration | 1 | 875.50 | 875.50 | 619.253 | < 0.001 | *** | |
| temperature | 1 | 1.30 | 1.30 | 0.896 | 0.349 | ||
| Algal species × algae concentration | 1 | 404.80 | 404.80 | 286.343 | < 0.001 | *** | |
| Algal species × temperature | 1 | 9.90 | 9.90 | 7.003 | 0.012 | * | |
| Algae concentration × temperature | 1 | 106.20 | 106.20 | 75.12 | < 0.001 | *** | |
| Algal species × algae concentration × temperature | 1 | 33.70 | 33.70 | 23.813 | < 0.001 | *** | |
| Residuals | 40 | 56.60 | 1.40 | ||||
| IRPI | Algal species | 1 | 0.00696 | 0.00696 | 48.194 | < 0.001 | *** |
| Algae concentration | 1 | 0.01844 | 0.01844 | 127.632 | < 0.001 | *** | |
| temperature | 1 | 0.00109 | 0.00109 | 7.541 | 0.009 | ** | |
| Algal species × algae concentration | 1 | 0.02120 | 0.02120 | 146.711 | < 0.001 | *** | |
| Algal species × temperature | 1 | 0.00108 | 0.00108 | 7.452 | 0.009 | ** | |
| Algae concentration × temperature | 1 | 0.00009 | 0.00009 | 0.654 | 0.424 | ||
| Algal species × algae concentration × temperature | 1 | 0.00039 | 0.00039 | 2.71 | 0.108 | ||
| Residuals | 40 | 0.00578 | 0.00014 | ||||
From the life table analysis, Alona guttata showed the longest ALS values when feeding on algal densities of 2×106 cells/mL (Fig. 3). Significant interactions were recorded for algal species×algal concentration (P<0.01), algal concentration×temperature (P<0.01), algae species×algae concentration×temperature (P<0.05) (Table 1). The ALS presented values from 26.97 (SD = 3.2752) to 53 d (SD = 1.8199), with N. oculata as the best food source for this chydorid when it was supplemented at 2×106 cells/mL.
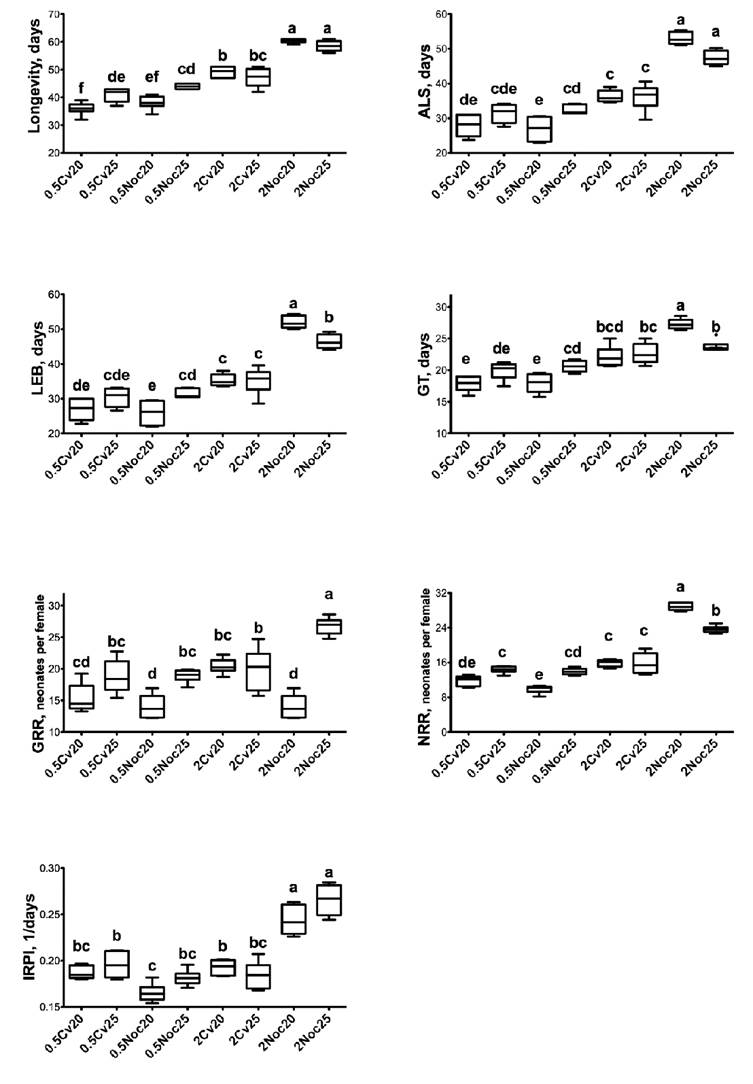
Figure 3 Demographic responses of Alona guttata fed on Chlorella vulgaris (Beijerinck, 1890) and Nannochloropsis oculata (Hibberd, 1981). ALS = average life span, GT = generation time, LEB = life expectancy at birth, GRR = gross reproductive rate, NRR = net reproductive rate, and IRPI = rate of population increase. All experiments were carried out with six replicates (n = 6). 0.5 = 0.5×106 cells/mL; 2 = 2×106 cells/mL; Cv = C. vulgaris; Noc = N. oculata; 20 and 25 = temperature in Celsius. Significant differences were established by factorial ANOVA and multiple comparison test of Bonferroni (P<0.05)
The life expectancy at birth (LEB) was mainly affected by the algal species and algae concentration; thus, obtaining the highest LEB value (52.50 d) with N. oculata (2×106 cells/mL) at 20ºC (Fig. 3). Significant interactions were registered for algae concentration×algal species, algae concentration×temperature, and for the three factors tested (Table 1).
The highest GT (27.28 ± 0.8018 days) was recorded when chydorids fed on N. oculata (2×106 cells/mL) and reared at 20ºC (Fig. 3). The lowest GTs were observed with the organisms fed on either C. vulgaris or N. oculata at 0.5×106 cells/mL. The two temperatures tested caused no significant differences in the GT of A. guttata, although their interactions significantly affected the GT (Table 1).
GRR was influenced by the algal concentration of both algal species (Table 1); then, organisms fed on 0.5×106 cells/mL produced fewer neonates than the organisms fed on 2×106 cells/mL. Thereafter, N. oculata at the highest algal density promoted A. guttata to have more neonates during their whole life cycle (Fig. 3).
The NRR registered the highest value with the females fed on N. oculata at 2×106 cells/mL (28.86 ± 0.8547 neonates/female), while those organisms fed on C. vulgaris produced fewer neonates, even when C. vulgaris was supplemented at 2×106 cells/mL (Fig. 3).
Finally, the treatment that promoted the higher rates of population increase was N. oculata at 2×106 cells/mL and temperature at 25°C (r = 0.2656 ± 0.0163). The fed on C. vulgaris did not reach the same population growth rates in comparison to the groups fed on N. oculata. Alona guttata fed on C. vulgaris exhibited similar growth rates than those females fed on N. oculata at 0.5×106 cells/mL (Fig. 3).
DISCUSSION
Although there are several reports on chydorid species, information on their life cycle or reproductive biology is still scarce. Therefore, the main contributions of this study are: a) the insights on A. guttata asexual reproduction since neither males nor ephipia were detected while carrying out the different experiments during this research; and b) the culture conditions that promoted the highest rates of population increase to obtain higher number of individuals for further usage, as food source for early life stages of fish (Alvarado-Suárez, 2017) or as test organisms in environmental toxicology (Garza-León et al., 2017; Osorio-Treviño et al., 2019).
The maximum survival of A. guttata was comprised within values reported for other chydorids (Table 2). The longest survival within the family Chydoridae were reported for organisms grown at 5°C, reaching values of about 100 d. In the subfamily Aloninae survival has registered values of up to 90 d. For instance, Cortez-Silva et al. (2022) reported that longevity of A. guttata reached 37 d as maximum (30.9 d average) when chydorids were fed on R. subcapitata, but we found that this species can survive longer when feeding on either C. vulgaris or N. oculata. It is worth pointing out that food source and temperature influenced longevity of organisms and several publications reported no more than one food source or temperature. Although several results showed long survival of chydorids, some others might be explored to improve the survival of organisms.
Table 2 Comparison of the life cycle and demographic responses of chydorids from this study and reported in the literature. GT, generation time (days); GRR, gross reproductive rate (neonates/female); NRR, net reproductive rate (neonates/female); IRPI, intrinsic rate of population increase; T, temperature
| Species | T (°C) | Survival (d) | GT | GRR | NRR | IRPI | Reference |
|---|---|---|---|---|---|---|---|
| Acroperus harpae (Baird, 1834) | 5 - 25 | 9 - 119 | 5.3 - 70.4 | Bottrell, 1975 | |||
| Alona affinis (Leydig, 1860) | 5 - 20 | 37 - 144 | 16.9 - 72.9 | Bottrell, 1975 | |||
| A. guttata (Sars, 1862) | 22 | 37 | Cortez-Silva et al., 2002 | ||||
| 20 - 25 | This study | ||||||
| A. iheringula (Sinev & Kotov, 2004) | 25 | 54 | 5.0 | Silva et al., 2014 | |||
| Alonella excisa (Fischer, 1854) | 73 | Sharma & Sharma, 1998 | |||||
| Chydorus pubescens (Sars, 1901) | 23.6 | 31 | 4.3 | Santos-Wisniewski et al., 2006 | |||
| C. sphaericus (Müller, 1776) | 5 - 20 | 23 - 96 | 8.9 - 38.5 | Bottrell, 1975 | |||
| 15 - 25 | 10.3 - 24.9 | 19.04 - 34.96 | 0.143 - 0.268 | Keen, 1967 (cited by Hann 1985) | |||
| Coronatella rectangula (Sars, 1862) | 25 | 26.92 - 30.82 | 10.2 - 10.6 | 18.0 - 18.8 | 19 - 27.12 | 0.25 - 0.26 | Muro-Cruz et al., 2002 |
| 23.6 | 28.4 | 4.2 | Viti et al., 2013 | ||||
| Disparalona rostrata (Koch, 1841) | 10 - 19 | 30 - 80 | 18.8 - 35.5 | Robertson, 1988 | |||
| Euryalona orientalis (Daday, 1898) | 28-30 | 24 | Venkataraman, 1990 | ||||
| Eurycercus lamellatus (Müller, 1776) | 5 - 20 | 42 - 162 | 19.1 - 100.5 | Bottrell, 1975 | |||
| E. longirostris (Hann, 1982) | 16 - 24 | 16 - 19 | 16.9 - 28.3 | 10.45 - 13.25 | 0.083 - 0.153 | Hann, 1985 | |
| E. vernalis (Hann, 1982) | 16 - 24 | 16 - 17 | 16.5 - 32.5 | 8.53 - 10.12 | 0.071 - 0.130 | Hann, 1985 | |
| 10 - 25 | 35.50 - 79.7 | 15.2 - 53.2 | 69 - 135 | 0.08 - 0.310 | Lemke & Benke, 2004 | ||
| Graptoleberis testudinaria (Fischer, 1851) | 5 - 20 | 23 - 95 | 9.5 - 44.6 | Bottrell, 1975 | |||
| Karualona muelleri (Richard, 1897) | 23 - 26 | 17 | 0.180 | Panarelli et al., 2019 | |||
| Leydigia acanthocercoides (Fischer, 1854) | 29 | 23 | 4.7 | Murugan & Job, 1982 | |||
| L. ciliata (Gauthier, 1939) | 28-30 | 46 | Venkataraman, 1990 | ||||
| L. leydigi (Schödler, 1863) | 5 - 19 | 21 - 120 | 10.1 - 63.9 | Robertson, 1988 | |||
| L. louisi mexicana (Kotov, Elías-Gutiérrez & Nieto, 2003) | 20 - 25 | 58 - 97 | 18.2 - 36.8 | 17.34 - 24.86 | 0.120 - 0.190 | Martínez-Jerónimo & Gómez-Díaz, 2011 | |
| Oxyurella longicaudis (Birge, 1910) | 23 | 47 | 7.50 | Castilho et al., 2015 | |||
| Pleuroxus aduncus (Jurine, 1820) | 25 | 16 - 44 | 7.4 - 14.4 | 1.04 - 13.8 | 6.47 - 0.53 | -0.091 - 0.149 | Nandini & Sarma, 2000 |
| P. denticulatus (Birge, 1879) | 15 - 25 | 8.4 - 25 | 4.92 - 10.96 | 0.096 - 0.174 | Keen, 1967 (cited by Hann 1985) | ||
| P. uncinatus (Baird, 1850) | 5 - 20 | 31 - 132 | 13.6 - 78.3 | Bottrell, 1975 |
The NRR in chydorids can take values from 6 to 135 neonates per female, with the highest reproduction rates in the genus Eurycercus (Table 2). In A. guttata the NRR was influenced by the algae concentration and the algal species, reaching the highest values at 2×106 cells/mL with N. oculata as food source. On this regard, Muro-Cruz et al. (2002) found no significant differences in the NRR of Coronatella rectangula (Sars, 1861) (formerly Alona rectangula) when feeding on C. vulgaris at 0.5 and 2×106 cells/mL (NRR = 14.72 ± 0.62 and 13.22 ± 0.77 neonates/female, respectively); however, A. guttata showed significant differences when fed on increasing algal concentrations, reaching higher reproductive rates, GRR and NRR, when organisms were fed on C. vulgaris or N. oculata at 2×106 cells/mL, which improved the reproductive performance of A. guttata.
The GT in chydorids varies significantly as a function of food source, food density, and temperature, taking values from some days (about 10 d) up to 100 d (Table 2). The longest GT were reported for organisms within the subfamily Chydorinae, like Acroperus harpae (Baird, 1834) and Eurycercus lamellatus (Müller, 1776) when they were grown at 5 °C, but increasing temperature to 25°C promoted significantly lower values of 5.30 d and 19.08 d, respectively. In the subfamily Aloninae, the longest GT was reported for Alona affinis (Leydig, 1860) (72.88 d at 5°C) while C. rectangula presented the shorter GT (4.16 d at 23.6°C) (Table 2). Thus, the GT of A. guttata (17.82 ± 1.15 to 27.28 ± 0.80 d) is similar to the values reported for other chydorid species. Bottrell (1975) stated that the interaction of abundant food supply and increasing temperature promote shorter times for every developmental stage in chydorids, thus, the GT can be shorten as it was observed within the group of A. guttata fed on N. oculata (2×106 cells/mL). On the other side, Muro-Cruz et al. (2002) found no significant differences for the GT of C. rectangula fed on C. vulgaris at either 0.5×106 or 2×106 cells/mL.
For chydorids, the intrinsic rate of population increase (r) is generally accepted to take low values, which are in most cases below or near 0.2/d (Martínez-Jerónimo & Gómez-Díaz, 2011; Nandini et al., 2007), with the exception of some species of the genus Eurycercus, which can reach high values for chydorids of up to 0.310/d (Table 2). Furthermore, the intrinsic rates of population increase are affected by temperature, for instance, Hann (1985) found that increasing temperatures promoted higher reproductive rates in chydorids by decreasing egg maturation times; therefore, in four species the highest “r” values were recorded at 24 - 25ºC. Despite A. guttata presented high growth rates in comparison to Pleuroxus aduncus (Jurine, 1820) 0.15/d (Nandini & Sarma, 2000), or very similar to those of C. rectangula or Karualona muelleri (Richard, 1897) (Muro-Cruz et al., 2002; Panarelli et al., 2019), such values are below the intrinsic growth rates that other cladocerans like Moina sp. can reach (Sipaúba-Tavares & Bachion, 2002; Deng & Xie, 2003; Rodríguez-Estrada et al., 2003).
In relation to algal density, some cladoceran species are adapted to either low or high densities; for instance, C. rectangula and P. aduncus showed lower growth rates at increasing algal densities (Nandini & Sarma,2000; Muro-Cruz et al. 2002). Nevertheless, these authors tested a single algal species. In our study, A. guttata exhibited higher growth rates with increasing algal density, with N. oculata as a better food source over C. vulgaris.
The algal species has also significant effects on the reproduction and survival of A. guttata. In this study we observed that either C. vulgaris or N. oculata were good food source, which promoted a higher performance than the alga R. subcapitata.
In the literature, the genera Chlorella and Nannochloropsis have been described to synthesize high amounts of polyunsaturated fatty acids (PUFA) in comparison to other genera like Raphidocelis (Patil et al., 2007); therefore, feeding on these algae could serve as a better food source that provides the essential nutrients to enhance reproduction and survival (Schlotz et al., 2012).
Up to date, three publications have reported the survival and fertility of A. guttata. On the one side, Garza-León et al. (2017) and Osorio-Treviño et al. (2019) carried out partial life table analysis with similar algal densities (N. oculata at 2×106 cells/mL) and temperature (25°C) than the present study; an on the other side, Cortez-Silva et al. (2022) studied the life cycle but used R. subcapitata as food source and temperature within the interval here tested. In these studies, neither of them employed any substrate in the culture media, and despite A. guttata survived and produced offspring in those conditions, we found out that the inclusion of substrate improved the performance of these chydorids, increasing their rate of population growth and survival. It has been demonstrated that not all chydorids grow better on a diet composed solely of microalgae but rather on one of detritus or bacteria (Smirnov, 1962; Vijverberg & Boersma, 1997), although a mixed diet could be more important for chydorids nutrition (Lemke et al., 2007).
In conclusion, A. guttata can feed on either C. vulgaris or N. oculata, increasing their longevity and fertility when algal density is high (2×106 cells/mL). Temperature had significant effects on the life history of A. guttata, but is was the interaction with the algal density and algal species which produced more significant effects on the performance of A. guttata. The continuous production of offspring and long survival in A. guttata allowed intrinsic growth rates that are among the highest within the family Chydoridae, which might suggest this species to be cultured for further purposes like aquaculture (since some Alona species form part of fish diets) or ecotoxicological studies, which have already been reported in the literature.











 nova página do texto(beta)
nova página do texto(beta)



