INTRODUCTION
In many countries the feeds for the rainbow trout (Oncorhynchus mykiss Walbaum, 1792) still rely on fishmeal (FM), a product derived from the marine fisheries (Tacon & Metian, 2008; Cid et al., 2020) and with the actual production fully utilized, the search of replacements for FM has been a top priority for the aquacultural industry (Gatlin et al., 2007). Plant origin products, such meals and oils of cereals, legumes, and oily seeds are economical alternatives to fish-origin products (Welker et al., 2018) and the protein concentrates are the most promissory ingredients to be included in salmonid feeds (Hardy, 2010). The plant protein concentrates such as those obtained from soy, rice, and corn, can be used to replace 75% of fishmeal in the diet of salmonids (Refstie et al., 2001). A total replacement of the fishmeal with the plant-origin concentrates in feeds might require the use of additives to improve the protein digestibility and the growth. Among such additives are the prebiotics (Guerreiro et al., 2018), non-digestible components that are metabolized by the intestine microbiota of the host (Ringø et al., 2010), which are reported to improve the growth performance (Munir et al., 2016) and the immunological responses (Dawood & Koshio, 2016).
Among several types of prebiotics, two are of the most used: the fructooligosaccharides (FOS) and mannanoligosaccharides (MOS). FOS are chains of b-D-fructans bounded by b-(2-1) glycosidic linkages (Ringø et al., 2010); while MOS are glucomanno-proteins derived from the cell wall of the yeast Saccharomyces cervisiae (Gainza & Romero, 2017). Their inclusion in diets has been reported for several species of fish (Yilmaz et al., 2007; Ringø et al., 2010; Dawood & Koshio, 2016; Guerreiro et al., 2018; Dawood et al., 2018), but limited information is available regarding their effects when plant-origin proteins are used: Guerreiro et al. (2015) reported the use of short chain FOS (scFOS) in a diet with soybean meal and other plant meals for the European seabass (Dicentrarchus labrax L. 1758), with no effect on growth or gut morphology. As well, Azedero et al. (2017) suggested that inclusion of scFOS to a diet with soybean meal in European seabass might have anti-inflammatory effect in the gut. Cid et al. (2020) reported that inclusion of FOS into a diet with plant-protein concentrates for juvenile rainbow trout, improved the growth performance and leucocytes count with respect of the control group fed with a commercial diet. Considering this, the objective of the present work was to evaluate the inclusion of FOS and MOS to diets with soy, rice and corn protein concentrates in the growth, blood serum biochemistry and intestine histology of juvenile rainbow trout.
MATERIALS AND METHODS
Experimental diets. A basal diet was formulated with protein concentrates of soybean (Estril-65, GABSA S.A. de C.V., Mexico; 64 ± 1 %, crude protein, dry weight basis), rice (GABSA S.A. de C.V., Mexico; 62 ± 2 %, crude protein, dry weight basis) and corn (Glutimex, Ingredion Mexico S.A. de C.V., Mexico; 59 ± 1.5%; crude protein, dry weight basis) as protein sources. The two experimental diets were prepared by adding either fructooligosaccharides (Forti-Feed P-95, Ingredion Mexico S.A de C.V., Mexico; D-FOS, 30 g/kg diet) or mannanoligosaccharides (Active-MOS, Ferpac International S.A. de C.V., Mexico; D-MOS, 30 g/kg diet) to the basal formulation (Table 1). As well, fish oil and soybean lecithin were added as lipid sources and dextrin as carbohydrate source. Besides a mixture of vitamins and minerals, wheat gluten was added as binder. a-cellulose was used to bring the diets up to 100%. The diets were prepared according with Sánchez et al. (2015) by mixing all the powered ingredients with the oils and water (40%) until obtain a wet dough. The dough was passed through a meat chopper (Model M-12-FS, Torrey S.A. de C.V., México) to obtain pellets of 5 mm of diameter. The diets were dried at 60°C in an inverted oven for 4 h and then, stored at -20°C until used. The basal diet corresponded to the control (D-Control), and a commercial diet (Grow Fish trucha 1, Malta Texo de México S.A. de C.V., México) was also used (Comm).
Table 1 Formulations and proximate composition of the diets fed to fingerlings of rainbow trout.
| Ingredients (g/kg) | D-Control | D-FOS | D-MOS |
|---|---|---|---|
| Soy protein concentrate | 234 | 234 | 234 |
| Rice protein concentrate | 241 | 241 | 241 |
| Corn protein concentrate | 170 | 170 | 170 |
| Cod liver oil | 70 | 70 | 70 |
| Soybean lecithin | 50 | 50 | 50 |
| Dextrine | 100 | 100 | 100 |
| Fructooligosaccharides | 0 | 30 | 0 |
| Mannanoligosaccharides | 0 | 0 | 30 |
| Mixture of vitamins and minerals1 | 40 | 40 | 40 |
| Wheat gluten | 50 | 50 | 50 |
| α-cellulose | 45 | 15 | 15 |
| Proximate composition | |||
| Moisture (%) | 8.1 ± 1.2 | 6.7 ± 1.0 | 5.6 ± 0.9 |
| Crude protein2 | 47 ± 3 | 47 ± 4 | 45 ± 2 |
| Crude lipids2 | 12 ± 1 | 13 ± 2 | 13 ± 1 |
| Ash2 | 5 ± 0.6 | 5 ± 0.8 | 5 ± 0.6 |
1Vitamin and mineral mixture (g/kg): ρ-aminobenzoic acid, 1.45; biotin, 0.02; myo-inositol, 14.5; nicotinic acid, 2.9; Ca-pantothenate, 1.0; pyridoxine-HCl, 0.17; riboflavin, 0.73; thiamine-HCl, 0.22; menadione, 0.17; α-tocopherol, 1.45; cyanocobalamine, 0.0003; calciferol, 0.03, L-ascorbyl-2-phosphate-Mg, 0.25; folic acid, 0.05; choline chloride, 29.65, NaCl, 1.838; MgSO4·7H2O, 6.85; NaH2PO4·2H2O, 4.36; KH2PO4, 11.99; Ca(H2PO4)2·2H2O, 6.79; Fe-citrate, 1.48; Ca-lactate, 16.35; AlCl3·6H2O, 0.009; ZnSO4·7H2O, 0.17; CuCl2, 0.0005; MnSO4·4H2O, 0.04; KI, 0.008 and CoCl2, 0.05.
2% dry weight basis
Experimental fish. Rainbow trout fingerlings of 60-days after hatching were acquired from the Centro de Producción Acuícola El Zarco, located in the municipality of Ocoyoacac, State of Mexico, Mexico. Fish were transported to the Laboratorio de Producción Acuícola of UNAM FES Iztacala and maintained in 500-L tanks provided with continuous filtration and aeration. The fish were fed on a commercial diet (Grow fish trucha 1, Malta Texo de México S.A. de C.V., México) until the start of the feeding trial.
Feeding trial. The feeding trial was conducted in a recirculation system with 12 polypropylene tanks of 100-L. Each tank was randomly stocked with 20 fingerlings with an initial weight of 1.75 ± 0.03 g (mean ± standard error) and each diet was fed to triplicate tanks. Every day, the fingerlings were fed at 7% of the total biomass of each tank and daily ration was divided into two equal feeding at 9:00 and 17:00 h. Every ten days, the fish were weighed, and the size of the ration was adjusted accordingly. During the trial, the water parameters were (mean± standard error): dissolved oxygen, 5.3 ± 0.1 mg/L; ammonia, 0.0 mg/L; pH 7.5 ± 0.2 y temperature of 16 ± 1°C. Water flow in each tank was of 1.5 L/min during the entire trial. All tanks were maintained under a natural cycle of 11 h light, 13 h darkness. The feeding trail was conducted for a period of 60 days.
At the end of the feeding trial, the organisms were starved for 24 h and weighed to obtain the growth performance. Then, 9 fish of each treatment were euthanized with an overdose of MS-222 (Sigma Aldrich Co., St. Louis, MO, USA) at 200 mg/L. Blood samples were obtained from the caudal vein for the determination of the protein, glucose and triglycerides in the serum. Then, samples of the liver and muscle were taken for the determination of the protein and lipids contents.
Growth performance. The parameters that were calculated are as follow:
Weight gain (%) WG= [(final weight - initial weight)/initial weight] x 100
Specific growth rate (%/day) SGR= [(ln final weight - ln initial weight)/days] x 100
Feed conversion efficiency FCE= weight gain (g)/total feed intake in dry weight basis (g)
Survival rate (%) = (initial number of fish/final number of fish) x 100.
Chemical analysis. The contents of protein in the diets, liver and muscle samples were analyzed according with AOAC (1990) by using a distillation unit (Kjeltec TM2100, Foss Analytics, Denmark). Lipids contents in the diets, liver and muscle were performed by the technique of Blight & Dyer (1959). The contents of moisture and ash in the diet were analyzed by the techniques reported by AOAC (1990).
Serum biochemistry. Samples of blood were allowed to clot for 2 h at 4 °C, then were centrifuged at 10,000 rpm during 10 min and the serum (supernatant) was collected and kept at -34°C until analyzed. The serum was analyzed for contents of protein (microBCA protein assay kit, Thermo Scientific, Rockford, IL, USA), glucose (glucose assay kit ab65333, abcam, Cambridge, UK) and triglycerides (EnzyChrom triglyceride assaykit ETGA-200, BioAssay Systems, Haywars, CA, USA).
Statistical analysis. Data of growth performance (WG, SGR, FER and survival), contents of protein and lipids in liver and muscle, concentrations of protein, glucose and triglycerides in blood serum were tested for normality and homoscedasticity with the Shapiro and Wilk W test and the Barletts´s test, respectively. As all data showed normality and homoscedasticity, a one-way ANOVA was performed using the Prism for Mac version 9.0 (GraphPad Software, San Diego, CA, USA). When found, significant differences among the treatments were determined by a Fisher LSD test with a significant level of 5% (p< 0.05) for each set of comparisons (Zar, 1999).
RESULTS
The growth performance (FW, WG, SGR, FCE and survival) is shows in Table 1. Inclusion of prebiotics in the diets with plant protein concentrates did not influence the growth of the fingerlings, as values of FW (p=0.6321), WG (p=0.8826) and SGR (p=0.9396) were lower on the groups fed with the D-FOS and D-MOS when compared with the D-Control, although no significant differences were observed. The lowest values were observed in the group fed the commercial diet. Regarding the survival rate, no significant differences (p=0.1414) were observed among the groups and the mortality registered was not conceived to be related to the treatments.
Liver protein content (Figure 1a) value of the fish fed the commercial diet was significantly higher (p=0.0369) than those observed for the fingerlings fed the diet D-control and D-MOS. Regarding the protein content in the muscle (Figure 1b) no significant differences (p=0.3594) were observed among all the groups.
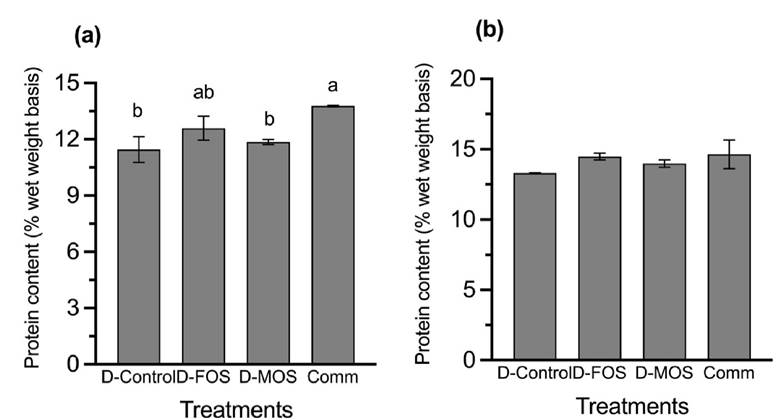
Figure 1a-b Protein contents of (a) liver and (b) muscle of rainbow trout fingerlings fed diets plant-origin concentrates and inclusion of the prebiotics FOS and MOS. Each bar represents the mean of triplicate groups ± standard error. Bars with different letters differ significantly (P<0.05).
In Figure 2a, lipid contents in the liver did not show significant differences (p=0.7261) among the treatments, while significant higher values (p=0.0588) were observed in the fish fed the D-MOS in the lipid content of muscle (Figure 2b).
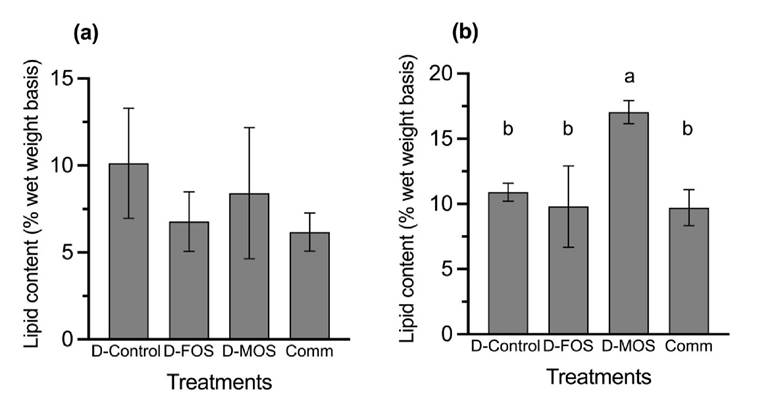
Figure 2a-b Lipid contents of (a) liver and (b) muscle of rainbow trout fingerlings fed diets plant-origin concentrates and inclusion of the prebiotics FOS and MOS. Each bar represents the mean of triplicate groups ± standard error. Bars with different letters differ significantly (P<0.05).
Contents of protein in serum (Figure 3a) were significantly higher (p=0.0671) in the fish fed iets with plant- protein concentrates when compared with those fed the commercial diet. Serum glucose contents (Figure 3b) did not show significant differences (p=0.7601) among the treatments. Serum triglycerides contents are shown in Figure 3c and significant higher values (p=0.0024) were observed in the group fed the commercial diet when compared with the rest of the groups.
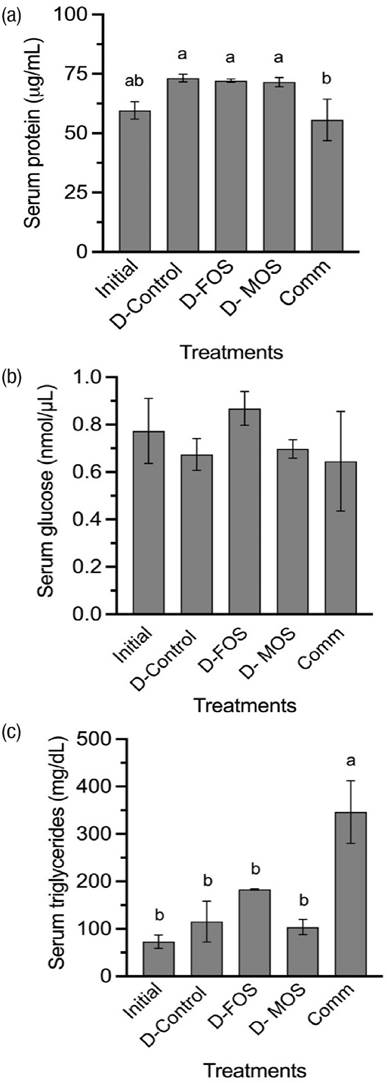
Figure 3a-c Serum contents of (a) protein, (b) glucose and (c) triglycerides of rainbow trout fingerlings fed diets plant-origin concentrates and inclusion of the prebiotics FOS and MOS. Each bar represents the mean of triplicate groups ± standard error. Bars with different letters differ significantly (P<0.05).
Table 2 Growth performance (final weight, FW; weight gain, WG; specific growth rate, SGR and feed conversion efficiency, FCE) and survival rate of rainbow trout fingerlings with diets plant-origin concentrates and inclusion of the prebiotics FOS and MOS. Each values represents the mean of triplicate groups ± standard error. Lines with different letters differ significantly (P<0.05).
| Treatments | ||||
| D-Control | D-FOS | D-MOS | Comm | |
| FW (g) | 8.1 ± 0.2 | 7.4 ± 0.4 | 7.6 ± 0.2 | 7.6 ± 0.4 |
| WG (%) | 354 ± 20 | 341 ± 34 | 328 ± 13 | 330 ± 33 |
| SGR (%/day) | 2.5 ± 0.07 | 2.4 ± 0.13 | 2.4 ± 0.05 | 2.4 ± 0.05 |
| FCE | 1.2 ± 0.02 | 1.3 ± 0.12 | 1.3 ± 0.15 | 1.1 ± 0.05 |
| Survival (%) | 91± 1 ab | 96 ± 3ab | 80 ± 10b | 100 ± 0a |
DISCUSSION
The search for sustainable sources of protein to replace the fish meal, has been a priority for the aquaculture industry in the last years (Gatlin et al., 2007). Plant protein sources has been recognized for many years as viable ingredients to be included in feeds for salmonids (Welker et al., 2018). We report the use of soy, rice and corn protein concentrates added with the prebiotics (FOS and MOS) for rainbow trout fingerlings.
Although prebiotics have reported to improve the growth of several species of fish (Yilmaz et al., 2007; Ringø et al., 2010; Dawood & Koshio, 2016; Guerreiro et al., 2018; Dawood et al., 2018), the present results showed that inclusion of the prebiotics did not improve the growth performance of the fingerlings compared with the organisms fed the D-Control and Comm. It seems that prebiotics do not have the same effect when plant-origin protein are used, as not growth improve was reported for the European seabass fed diets with soybean meal and added with scFOS (Guerreiro et al., 2015) and in rainbow trout fed diet with grain distiller dried yeast and MOS (Betiku et al., 2018). According to Dawood & Koshio (2016) prebiotic efficiency depends on the type, concentration in the diet, the fish species, and length of period of feeding. As well, Guerreiro et al. (2018) reported that diet composition might affect the efficiency of the prebiotic, but so far there is not a clear explanation of lack of effect when plant ingredients are used.
Contents of protein and lipid were assessed in muscle and liver. Reports of the effects of prebiotics on protein and lipid contents are limited: Yilmaz et al. (2007) reported that MOS increased the protein content in carcass of rainbow trout; while Cid et al. (2020) reported increased values of protein contents of rainbow trout fingerlings fed with a plant protein-based diet added with FOS. In this study, the muscle protein content was similar among the groups, regardless the inclusion of prebiotics. In the case of liver, protein contents of the fish fed the diets with the plant protein concentrates showed lower values than that observed for the fish fed the commercial diet. However, the liver contents of protein were between 11 and 13%, which are similar to the 10 to 12.5% reported for Aguillón et al. (2017) and 12 to 17% found by Cid et al. (2020), both studies used with diets based on plant proteins. The muscle lipid contents were higher to those previously reported in rainbow trout fingerlings fed different plant protein ingredients (Aguillón et al., 2017; Cid et al., 2020) and according with Carrillo et al. (2018), such contents might be related to their use as energy source for muscle. Regarding the lipid contents in the liver, the tendency of higher values found in the groups fed diets with plant protein concentrates has been reported previously (Carrillo et al., 2018). According with Aguillón et al. (2017), the inclusion of taurine to plant-protein based diets has a hypolipidemic effect and due to an increase of the bile acid that leads to a higher activity of the lipase (Chatzifotis et al., 2008). The diets with the plant protein concentrates were no added with taurine, which might influence the higher lipid content in liver. However, more research is necessary to understand the effects of the plant protein concentrates on the lipid metabolism.
Blood serum biochemistry offers a rapid method to assess the organism physiology, wellness and it is widely used as a diagnostic tool (Manera, 2021). Manera & Britti (2006) reported values of 35.9 mg/mL of serum protein for juvenile rainbow trout, which are similar to those found in the present work (Figure 3a). The observed levels of protein in the groups fed the diets with the plant concentrates might indicate proper dietary protein utilization and normal hepatic function, as it has been reported that use of plant-origin protein usually cause abnormal lower values of serum protein (Iqbal et al., 2021; Abdel-Tawwab et al., 2021). Values of serum glucose, in the other hand, were similar to the 1.08 nmol/mL reported by Manera & Britti (2006) and 0.96 nmol/mL reported by Hernández et al. (2016) for juvenile and fingerlings of rainbow trout, respectively. Regarding the triglycerides in serum, the normal contents of rainbow were reported to be 347.5 mg/L (Manera & Britti, 2006) and the organisms fed the commercial diet were found similar. However, the initial sample and the organism fed with the plant protein concentrates diets showed lower values. According with the manufacture information, the commercial diet had a minimum lipid content of 16%, around 3% more that the diets with plant-origin proteins and seems to influence the level of triglycerides in the serum. The use of the experimental diets with the concentrates, regardless the inclusion of prebiotics, seems to not affect the normal serum parameters in the rainbow trout fingerlings.
In conclusion the diets based on soy, rice and corn protein concentrates and the inclusion of FOS or MOS, showed no effects in the growth performance and serum total protein and glucose of rainbow trout fingerlings. Regardless inclusion of FOS and MOS in the diet, the present results indicate the possibility to use the formulations without affecting the growth and wellness of rainbow trout fingerlings, but more research is necessary regarding the possible effects of lipids on the liver and muscle.











 nueva página del texto (beta)
nueva página del texto (beta)



