INTRODUCTION
The Parque Nacional Sistema Arrecifal Veracruzano (PNSAV) is a highly productive ecosystem (Rodríguez-Gómez et al., 2013), which is the reason it was designated as a Marine Protected Area under the classification of National Marine Park in 1992 (DOF, 1992, 2012) and has since been declared a Biosphere Reserve within the Man and the Biosphere Program (UNESCO, 2006) and registered as a Ramsar site (number 1346) (FIR, 2004). This reef system is comprised of several individual coral reefs, divided into northern and southern groups by the influence of the fluvial plume of the Jamapa River (Horta-Puga et al., 2016).
Coral reef ecosystems such as PNSAV show an intricated architectural complexity (Jordán-Dahlgren, 2004), and support benthic and planktonic invertebrate assemblages of high biodiversity (Horta-Puga et al., 2007).Some of the zooplankton biota found in the PNSAV have been studied, such as appendicularians, chaetognaths, pteropods, copepods, as well as fish species (Flores-Coto, 1965, 1974; Leal-Rodríguez, 1965; Vega-Rodriguez, 1965; Aguayo-Saviñon, 1966; Campos, 1980; 1980; Suárez, 1992; Campos-Hernández & Suárez-Morales, 1994; Ayala-Rodríguez et al., 2016). Okolodkov et al. (2011) conducted a study on the biomass of plankton, reporting that invertebrates are the most abundant group of zooplankton in this reef system. Cházaro-Olvera et al. (2019) found that 13.77% of the community of zooplankton were decapods, including commercial species such as Callinectes sp., Menippe sp., and Penaeus sp. Around 259 species of Crustacea have been found in the PNSAV, nine species belonging to suborder Dendrobranchiata (Hermoso-Salazar & Arvizu-Coyotzi, 2015).
The PNSAV is located in the southern Gulf of Mexico and this region supports an important shrimp fishery based on three species of Dendrobranchiata: the brown shrimp, Penaeus aztecus Ives, 1891, the white shrimp, P. setiferus Linnaeus, 1767, and the pink shrimp, P. duorarum Burkenroad, 1939 (Gracía & Soto, 1990; Gómez-Ponce et al., 2018).
The shrimp fishery is the fourth most important by catch volume in the Gulf of Mexico. Almost 80% of total capture corresponds to P. aztecus, caught mainly in theTamaulipas and Veracruz states; in the latter, between the years 2004 and 2013, up to 10% of Gulf of México total catch was obtained (INP, 2014).
All Penaeus spp. stages are free living, the three commercially important penaeids spawn eggs in coastal waters, usually at depths of <50 m. The larvae become planktonic after hatching and transitioning through a series of stages, i.e., nauplius, zoea (including protozoea and mysis stages), and later, as tidal and wind-driven currents carry the larvae shoreward, the decapodid and juvenile stages eventually enter the estuary, become demersal, and move into coastal marshes to feed and grow before returning to shelf waters as sub-adults (Ditty & Alvarado, 2011; Martin et al., 2014). Larval development is regular anamorphic (Anger, 2001), meaning that the appearance of characters and shape changes are gradual.
The brown shrimp, P. aztecus, typically occur from the west of the Mississippi River Delta to the Mexican State of Tamaulipas, however, they have also been caught from Martha’s Vineyard, Massachusetts, to the Florida Keys, and in the west and south of the Gulf of Mexico, from Apalachicola Bay, Florida, to the northwestern coast of the Yucatan Peninsula, Mexico (Felder & Camp, 2009). Spawning peaks of P. aztecus occur from December to April (Zimmerman & Minello, 1984; Rozas & Reed, 1993; Matthews, 2008) and a smaller, secondary peak occurs during late summer and early fall (Rogers et al., 1993).
The pelagic larval stage dispersion is considered to be an advantage for this species because it enhances genetic flow and the colonization of new areas.
The transport of penaeid larvae and postlarvae is a complex process that includes mechanisms such as selective tidal transport induced by synchronized salinity changes (Hughes, 1969), endogenous rhythms (Hughes, 1972), or hydrostatic pressure (Forbes & Benfield, 1986; Rothlisberg et al., 1995). Another hypothesis suggests that changes in coastal water temperature, salinity, and the direction of currents, in combination with the diel movements of larvae and postlarvae, facilitate their transport (Hughes, 1969; Rogers et al., 1993). It has also been suggested that vertical migration, tidal and wind-forced currents are a possible mode of recruitment (Rothlisberg et al., 1983; Wenner et al., 1998).
To this day, larval transport and recruitment research has reached considerable progress, for example, on pre-settlement stages as their entrance to inshore habitats is related to biological and oceanographic variables.Despite these advances, knowledge about the larval recruitment of tropical fish species and invertebrates in the Caribbean Sea and Gulf of Mexico is scarce (Criales et al., 2002), due to this in this contribution were analyzed the distribution and abundance of P. aztecus mysis stage and the relationships to environmental factors in PNSAV.
MATERIAL AND METHODS
The PNSAV is located on the continental shelf of the state of Veracruz, off the coast of Boca del Río and Alvarado municipalities, in the southern Gulf of Mexico (19°00’00”-19°16’00”N, 95°45’00”-96°12’00”W). The park consists of 23 reef banks divided by the estuarine inlet of the Jamapa River; 12 reefs are in front of the port of Veracruz (northern group) and 11 more are in front of the Municipality of Antón Lizardo (southern group). In total, they occupy an area of 52,283 hectares. The park presents islands and platform reefs (Granados-Barba et al., 2007; Horta-Puga et al., 2007).
The Gulf of Mexico is in a transition area; therefore, seasonal variability is less pronounced in the southern tropical half. For that reason, the climate in the PNSAV cannot be divided seasonally; instead we used dry and rainy seasons and cold fronts (Carrillo et al., 2007). Cold fronts are anticyclonic cold wind currents that enter the Gulf of Mexico from North America, generating strong northern winds, with occasional precipitation and temperature drops (Carrillo et al., 2007; Ojeda et al., 2017), this season occurs from October to March. The ‘dry weather conditions’ occur from May to June, with scarce rainfall and higher temperatures. The ‘rainy weather conditions’ occur from July to September, when temperatures and precipitation increase and winds are weaker (Carrillo et al., 2007; Zavala-Hidalgo et al., 2014). The average annual temperature in the reef zone is 26°C (Chávez et al., 2007).
Field work. Samples were collected under cold front and rainy weather conditions in the years 2011, 2012, and 2013. P. aztecus larval stages were obtained in 26 sampling stations located in four transects, two transects northward and another two transects southward of the Jamapa river estuarine inlet (Fig. 1).
Horizontal surface hauls were made on the stations using a 1.5 m-long conical net WP2 with a 0.5 m mouth diameter (surface = 0.196 m2) and 330 μm mesh opening with a flowmeter (General Oceanics) to determine the volume of filtered water. The hauls were conducted from a boat with an outboard motor and lasted for 5 min at an average speed of 3 knots (1.543 m s−1), equivalent to an approximate distance of 450 m and water volume of 350 m3 for each sampling site. Samples were concentrated and fixed in 500 ml flasks with 10 ml of 4% formaldehyde and neutralized with sodium borate. In situ measurements included salinity, surface temperature of water (°C), and dissolved oxygen (mg l−1) which were measured using a multiparameter water quality portable meter (Hanna HI 9828).
Laboratory work. The biological material was transferred to the Crustacean Laboratory at the Facultad de Estudios Superiores Iztacala of the Universidad Nacional Autónoma de México. Samples were transferred to 70% alcohol 24 h after fixation. Larvae and decapodids species sorting was carried employing Calazan (1993) and Dos Santos & Lindley (2001) identification keys. A Motic SMZ-168 stereoscopic microscope and a Leica DM750 microscope were used. The total number of individuals per species was counted and mysis density was standardized to the number of mysis per 100 m3 (Suárez-Morales & Gasca, 2000).
Statistical analysis. The generalized least squares (GLS) model was used to compare temperature (°C), salinity, and dissolved oxygen (mgl-1) under cold front (Cf) and rainy (R) weather conditions (Wc) during three sampling years (2011, 2012, and 2013), transects (Northern, N; North central, Nc; South central, Sc; Southern, S) and shore zones (Fs, Foreshore; Ns, Norshore; Offs, Offshore) (Chowdhury & Behera, 2019) (Zuur et al., 2007). GLS was performed using SPSS v25.
To test whether the density of the species changed as a function of weather conditions and sampling zones, a two-way factorial design was performed. Zones (three) and weather conditions (two) were considered as fixed orthogonal factors. This design was applied to analyze the density of zoeae using a permutational multivariate analysis of variance (PERMANOVA) test. The values of environmental parameters were arcsine transformed and normalized. The values of the density of the species were transformed into the Log (n+1). The resemblance matrix for the density of the species was achieved using the Bray-Curtis similarity (Clarke & Gorley, 2016). The analysis was computed with 9999 permutations of residuals in a reduced model. Pairwise tests using a t-statistic were used to identify differences between weather conditions, latitudinal zones and shore zones. PERMANOVA were performed as implemented in the PRIMER V7 and PERMANOVA add-ons (Anderson et al., 2008; Clarke & Gorley, 2016).
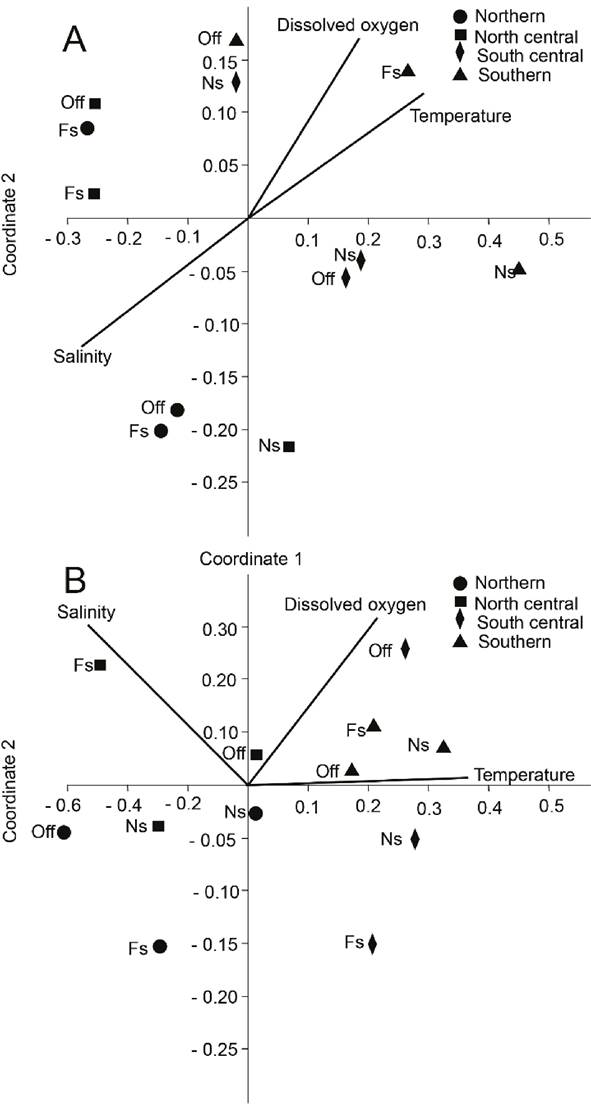
The relationships among P. aztecus mysis densities and water temperature, salinity and dissolved oxygen mean values were explored with Non-metric multidimensional scaling ordination method (NMDS), using a resemblance matrix constructed with the Bray-Curtis index. NMDS was performed using PAST software (Hammer et al., 2001).
RESULTS
Environmental factors. In rainy weather conditions, a temperature variation was determined between 27.49 ± 0.46 °C in north central-Offs and 29.98 ± 0.02 °C in southern-Fs; in weather conditions associated with cold fronts the temperature varied between 23.21 ± 0.05 °C in north central-Fs and 25.74 ± 0.37 °C in southern-Offs (Fig. 2A). The water temperature showed significant differences related to transect location (F(3,61) = 7.310, p < 0.001), shore zone (F(2,61)= 79.757, p < 0.001), and weather condition (F(5,61) = 322.07, p < 0.001). Differences were also found when comparing the northern and north central transects to the southern and south central transects, the three shore zones, and the weather conditions (p < 0.05) (Table 1A).

Figure. 2 Relation between the zone of sampling and environmental factors. A, temperature °C; B salinity (psu); C, dissolved oxygen mgL-1. Black circles, rainy weather conditions; Black squares, cold fronts weather conditions.
Table 1 Model Generalized Least Squares applied to compare the temperature (°C), salinity (psu) and dissolved oxygen (mgl-1) from transects (Tr) and shore zones (Sz) under weather conditions (Wc) for each sampling year. *, significant differences. Cf, cold fronts; Comp, comparison between transects, weather conditions and shore zones; Fs, foreshore; Offs, offshore; N, northern; Nc, north central; Ns, nearshore; R, rainy; S, southern; Sc, south central.
| Temperature | |||||||
| Source | SC | Gl | Mc | F | p | Comp | p (Tukey) |
| Corrected model | 178.22a | 10 | 17.82 | 179.15 | < 0.001* | N-Nc | 1.000 |
| Intersection | 69363.56 | 1 | 69363.56 | 697255.67 | < 0.001* | N-Sc | 0.004* |
| Tr | 2.18 | 3 | 0.73 | 7.31 | < 0.001* | N-Sc | 0.016* |
| Sz | 15.87 | 2 | 7.93 | 79.76 | < 0.001* | Nc-Sc | 0.005* |
| Wc | 160.17 | 5 | 32.03 | 322.02 | < 0.001* | Nc-S | 0.021* |
| Residual | 6.07 | 61 | 0.09 | Sc-S | 0.956 | ||
| Total | 69547.85 | 72 | Cf-R | <0.001* | |||
| Corrected total | 184.29 | 71 | Fs-Ns | <0.001* | |||
| r2 = 0.97 | Fs-Offs | <0.001* | |||||
| Ns-Offs | <0.001* | ||||||
| Salinity | |||||||
| Source | SC | Gl | MC | F | p | Comp | p (Tukey) |
| Corrected model | 18.01a | 10 | 1.80 | 17.87 | < 0.001* | N-Sc | 0.014* |
| Intersection | 93851.66 | 1 | 93851.66 | 931188.30 | < 0.001* | N-S | <0.001* |
| Tr | 2.82 | 3 | 0.94 | 9.32 | < 0.001* | Nc-Sc | 0.246 |
| Sz | 6.19 | 2 | 3.09 | 30.71 | < 0.001* | Nc-S | 0.003* |
| Wc | 8.99 | 5 | 1.8 | 17.86 | < 0.001* | Sc-S | 0.287 |
| Residual | 6.15 | 61 | 0.10 | Cf-R | 0.001* | ||
| Total | 93875.82 | 72 | Fs-Ns | <0.001* | |||
| Corrected total | 24.16 | 71 | Fs-Offs | <0.001* | |||
| r2 = 0.75 | Ns-Offs | <0.001* | |||||
| Dissolved oxygen | |||||||
| Source | SC | Gl | MC | F | p | Comp | p (Tukey) |
| Corrected model | 66.91a | 10 | 6.69 | 20.77 | < 0.001* | N-Nc | <0.001* |
| Intersection | 11720.09 | 1 | 11720.09 | 36378.88 | < 0.001* | N-Sc | <0.001* |
| Tr | 45.88 | 3 | 15.29 | 47.47 | < 0.001* | N-S | <0.001* |
| Sz | 10.42 | 2 | 5.21 | 16.16 | < 0.001* | Nc-S | <0.001* |
| Wc | 10.61 | 5 | 2.12 | 6.59 | < 0.001* | Sc-S | <0.001* |
| Residual | 19.652 | 61 | 0.32 | Cf-R | <0.001* | ||
| Total | 11806.65 | 72 | Fs-Ns | 0.019* | |||
| Corrected total | 86.56 | 71 | Fs-Offs | <0.001* | |||
| r2 = 0.773 | Ns-Offs | 0.014* |
Salinity in rainy weather conditions was 32.18 ± 0.06 in southern-Fs and 35.40 ± 0.52 in the northern-Offs; in cold front weather conditions, the salinity was 34.59 ± 0.39 in the south central-Fs compared to 35.84 ± 0.17 in the northern-Offs (Fig. 2b). Salinity Showed significative differences depending on transect (F(3,61) = 9.324, p < 0.001), shore zone (F(2,61)= 30.705, p < 0.001), and weather conditions (F(5, 61) = 17.858, p < 0.001). Northern transect salinity had significant differences with respect to the southern and south central transects (but not with north central transect), and the north central transect salinity showed significant differences with respect to the southern transect only. These salinity differences were found among the three shore zones and the two weather conditions (p < 0.05) (Table 1B).
In rainy weather conditions the dissolved oxygen level was 3.64 ± 0.13 mgL-1 in the northern-Fs to 6.18 ± 0.64 mgL-1 in the southern-Offs; in cold front weather conditions dissolved oxygen ranged from 4.04 ± 0.06 mgL-1 in the north central-Fs to 6.02 ± mgL-1 in the southern-Offs (Fig. 2c). The dissolved oxygen concentrations showed significant differences depending on transect(F(3,61) = 47.474, p < 0.001), shore zone (F(2,61)= 16.162, p < 0.001), and weather conditions (F(5,61) = 6.588, p < 0.001). Differences in dissolved oxygen concentrations were found between the northern and the southern and south central transects, the three shore zones, and between cold front conditions in 2011 and 2013 with rainy conditions in 2011, and between cold front weather conditions in 2012 with rainy conditions in 2011 and 2012 (Tukey p < 0.05) (Table 1C).
Mysis density. The highest average density, 6,938 ± 326 mysis 100 m−3, was found during rainy season in 2011 in the southern transect-Ns, followed by 3,321 ± 339 mysis 100 m−3 in the south central-Offs transect during cold front weather conditions in 2013, and 3,056 ± 236 mysis 100 m−3 in the south central-Fs transect during cold front weather conditions in 2011 (Table 2). According to the PERMANOVA results, the P. aztecus mysis density changed according to weather conditions (F(5,54) = 2.73, p = 0.025) considering the distance off the coastline. The pairwise comparison test revealed that differences in mysis density were apparent among 2013 cold front season and 2012 and 2013 rainy seasons (p < 0.05).
Table 2 Penaeus aztecus. Density of mysis100 m−3(± SD) in the PNSAV during rainy and cold fronts of 2011, 2012 and 2013. Cold fronts, Cf; Shore zone, Sz; Rainy, R. Bold highest density.
| Transect | Sz | R-2011 | Cf-2011 | R-2012 | Cf-2012 | R-2013 | Cf- 2013 |
|---|---|---|---|---|---|---|---|
| Northern | Foreshore | 208 ± 36 | 40 ± 12 | ||||
| ig | Noreshore | 72 ± 15 | 28 ± 8 | 184 ± 22 | 660 ± 113 | ||
| North central | Foreshore | 35 ± 7 | 80 ± 25 | ||||
| North central | Noreshore | 8 ± 2 | 9 | 43 ± 18 | 72 ± 21 | ||
| North central | Offshore | 40 ± 12 | 33 ± 10 | 225 ± 25 | 2704 ± 356 | ||
| South central | Foreshore | 49 ± 14 | 3056 ± 236 | 56 ± 16 | 56 ± 14 | 113 ± 26 | |
| South central | Noreshore | 256 ± 36 | 666 ± 125 | 159 ± 21 | 1439 ± 189 | ||
| South central | Offshore | 139 ± 17 | 272 ± 56 | 3321 ± 339 | |||
| Southern | Foreshore | 1682 ± 125 | 20 ± 6 | 26 ± 6 | 248 ± 65 | 13 ± 3 | 464 ± 89 |
| Southern | Noreshore | 6938 ± 326 | 112 ± 26 | 59 ± 12 | 728 ± 112 | 555 ± 145 | |
| Southern | Offshore | 64 ± 11 | 59 ± 16 | 55 ± 18 | 88 ± 12 | 872 ± 258 |
Respect to the transects, changes were found among weather conditions (F(3,48) = 5.18, p = 0.004) and transects too (F(5,48) = 3.38, p = 0.009). The pairwise comparisons revealed that mysis density was different among 2013 cold fronts and 2012 and 2013 rainy season cfonditions, also among the 2011 cold fronts season conditions and 2012 rainy season conditions (p < 0.05). Furthermore, the pairwise comparisons showed differences among northern transect, southern transect and south-central transect; another difference was among north-central transect and the southern transect (p < 0.05) (Table 3).
Table 3 Permutational multivariate analysis of variance (PERMANOVA) on the density of mysis of Penaeus aztecus from PNSAV reef, based on an orthogonal two-factors model. Cf, cold fronts; Comp, comparison between transects, weather conditions and shore zones; N, northern; Nc, north central; S, southern; Sc, south central; Sz, Shore zones; R, Rainy; Tr, Transects, Wc, Weather conditions. *, significant differences (p < 0.05).
| Shore zones | |||||||
| Source | SS | df | MS | F | P | Comp | p (Tukey) |
| Sz | 0.52 | 2 | 0.26 | 1.12 | 0.312 | Cf2013-R2012 | 0.001* |
| Wc | 3.14 | 5 | 0.63 | 2.73 | 0.025* | Cf2013-R2013 | 0.031* |
| Interaction | 1.54 | 10 | 0.15 | 0.67 | 0.766 | ||
| Residual | 12.43 | 54 | 0.23 | ||||
| Total | 17.63 | 71 | |||||
| Transects | |||||||
| Source | SS | df | MS | F | P | Comp | p (Tukey) |
| Tr | 2.8918 | 3 | 0.96394 | 5.18 | 0.004* | N-S | <0.001* |
| Wc | 3.1443 | 5 | 0.62885 | 3.38 | 0.009* | N-Sc | 0.016* |
| Interaction | 2.655 | 15 | 0.177 | 0.95 | 0.513 | Nc-S | 0.011* |
| Residual | 8.9383 | 48 | 0.18621 | Cf2011-R2012 | 0.039* | ||
| Total | 17.629 | 71 |
The NMDS results identified two groups in rainy seasons, separated by transects (stress = 0.117); one group comprising the northern and north central zones, with low densities and average temperature of 28.68 ± 0.76 °C, salinity of 34.58 ± 0.64 and dissolved oxygen concentration of 4.43 ± 0.86 mgL-1;the second group, with higher densities, was comprised by the southern and south central transects, with average temperature 29.35 ± 0.56 °C, salinity of 33.66 ± 0.94 and dissolved oxygen levels of 5.68 ± 0.39 mgL-1 (Fig. 3a). The NMDS analysis also separated two groups (stress = 0.043) in cold front weather conditions: a group in the northern and north central zones, with low densities and average temperature of 24.41 ± 0.73 °C, salinity 35.32 ± 0.47, and dissolved oxygen levels of 4.16 ± 0.17 mgL-1; the second group, with higher densities, was formed by the southern and south central transects, with average temperature of 24.44 ± 1.08 °C, salinity of 35.19 ± 0.47, and dissolved oxygen concentration of 5.01 ± 0.71 mgL-1 (Fig. 3b).
DISCUSSION
Changes in environmental conditions in the PNSAV region during periods of weather associated with a cold front, rainy weather, tropical storms, and hurricanes cause a mixed layer in the water column, incorporating nutrients into the photic zone (Zavala-Hidalgo et al., 2006, 2014), which can be used by phytoplankton, zooplankton such as mysis that feed on the algae (Schwamborn et al., 2001). In cold front weather conditions winds along Tamaulipas and Veracruz coasts produce cyclonic currents in a southerly direction, while those in the states of Campeche Bank and Yucatán form anticyclonic currents. These currents converge at the southern end of the Gulf of Mexico, forming flows, perpendicular to the coast, from the neritic-oceanic zone to the oceanic zone (Zavala-Hidalgo et al., 2006). Furthermore, the subtropical water underlying the Campeche Bank enhances the resuspension of nutrient-rich sediments in the southern part of the PNSAV. Rodríguez-Gómez et al. (2015) found that the highest levels of chlorophyll and, consequently, of gross primary productivity in the PNSAV begin to appear in September and at the end of October to April. Thus, the highest mysis density obtained in this study under rainy weather conditions could be associated to the values of dissolved oxygen, due to the increase in chlorophyll (Álvarez-Cadena et al., 2007), and a response to nutrient input by the estuarine plume of the Jamapa River during rainy conditions (Horta-Puga et al., 2016). However, Landeira & Lozano-Soldevilla (2018), commented that valuable information is obtained from knowing the place and potential spawning season for a species, and the temporal distribution of decapod larvae suggests a larval hatching period for many species with peaks of abundance associated with seasonality. So, peak spawning from December through April (Zimmerman & Minello, 1984; Rozas & Reed, 1993; Matthews, 2008) and a second peak that occurs during late summer and early fall (Rogers et al., 1993) is consistent with the peak of abundance obtained in this study in rainy weather and the continuous presence of high mysis abundance in cold front weather conditions.
The GLS and PERMANOVA tests allow the distinction of two zones: northern zone and southern zone, related to the freshwater inflows and sediment loadings from Jamapa river. However, is important to point out than during cold fronts weather conditions, the inshore marine current turns to a southward direction and Jamapa river plume reaches its highest influence over the nearest reefs to Anton Lizardo coast; in contrast, during dry weather conditions the inshore marine currents go northward and the Jamapa river plume change its influence over the reefs ubicated in front of Veracruz Port (Krutak et al., 1980).
The multivariate analysis (NMDS) results also distinguished two zones, based on dissolved oxygen concentration, since the northern zone had low concentrations (up to 4 mgL-1) while the northern zone had higher values (up to 5 mgL-1). According to the EPA (1986) and the ecological Criteria of the European Community (1989), the concentration of dissolved oxygen needed for the protection of aquatic life in both fresh water and marine water is 5 mg L-1. Some aquatic animals can tolerate a lower concentration of dissolved oxygen for a period; however, this has negative effects on biodiversity, growth, and reproduction.
Regarding density, Cházaro-Olvera et al. (2009), found values of 146,530 postlarvae 100 m-3 in the tidal flow of an estuarine lagoon inlet; Wenner et al. (2005) found that the density of L. setiferus ranged from 1,300 postlarvae 100 m-3 to 29,900 postlarvae 100 m-3 in surface hauls during night-time flow tides. In the present study we obtained approximately 7,000 mysis 100 m-3 in the nearshore zone in daytime surface hauls. Thus, in open coastal systems dispersion of larvae and postlarvae is higher than in channels where larvae enter estuarine systems.
Finally, with respect to P. aztecus life history, Darnell et al. (1983) and Neal et al. (1983) mentioned that adults reproduce in marine environmental conditions beyond the 18 m depth, and the spawning sites are deeper and far away from shore (Williams, 1984). But the larval distribution, nauplii, protozoea, and mysis stages are found nearby the coast (Sick, 1970; Zimmerman & Minello, 1984), mainly from September to April (Sandifer, 1973; Williams, 1984). The results showed in this study are consistent evidence of previous reports, referred to highest density of P. aztecus mysis was found during rains and cold fronts weather conditions and in the foreshore zone, in this case in the south area of the PNSAV.











 nueva página del texto (beta)
nueva página del texto (beta)