INTRODUCTION
The invasion of lionfish Pterois volitans Linnaeus, 1758 and P. miles (Bennett, 1828) in the northwest Atlantic and the Caribbean is one of the fastest invasions of marine fish recorded in history (Morris et al., 2008). These species are considered as established in these seas (Morris et al., 2009; Schofield, 2009; Schofield, 2010).
Lionfish are a major concern for managers of marine protected areas, governments, and local communities, and their impacts as predators affect the reef fauna, particularly the fish community (Albins & Hixon, 2008). P. volitans is far more abundant than P. miles; therefore,current research is focused on the former species. It has been determined that the lionfish is able to cause a 79% reduction in the recruitment of forage fish (Whitfield et al., 2007), which play a key role contributing to maintain the equilibrium in the algae-coral relationship. Meanwhile, Green et al. (2012a) reported biomass reductions in 65% of 42 species of small reef fish. Other authors have also documented the predatory impact of this species on the reef (Green & Côté, 2009; Brown et al., 2009; Santander-Monsalvo et al., 2012). From the economic viewpoint, impacts of lionfish are expected on fisheries and in the reduction of reef fish that are major attractions for tourism, thus deserving evaluation.
The first confirmed record of lionfish in Atlantic waters occurred in Florida, where a specimen was collected in October 1985 (Morris & Akins, 2009). Since 1992, lionfish were observed in Palm Beach, Boca Raton, and Miami, Florida; since 2000, they were recorded in North Carolina, South Carolina, Georgia, and Bermuda (Whitfield et al., 2002; Hare & Whitfield, 2003; USGS, 2004; Reef, 2008). The species continued to expand rapidly in the Atlantic and the Caribbean (Schofield, 2009) as well as in the Gulf of Mexico (Brown & Ruiz-Carus, 2006; Aguilar-Perera et al., 2012; Santander-Monsalvo et al., 2012).
In Cuba, lionfish were first reported in 2007 off the coast of Santiago de Cuba, near the Aquarium of Baconao (Chevalier et al., 2008), and rapidly colonized the rest of the coast, to the extent that since 2010, it was considered as a well-established species in the country.
The seasonality of lionfish reproduction throughout its natural range is unknown (Morris et al., 2008). Ruiz-Carus et al. (2006) stated that this species may have been breeding in Florida during the first months of the year; their rapid colonization of other areas led to suspect that reproduction may occur throughout the year under suitable conditions (Morris et al., 2009).
Therefore, this study was designed to fulfill the scarce information on the breeding process of lionfish in Cuba and the need to address recommendations to control their populations aiming to minimize their impacts on local reefs. Specific goals were to determine (1) seasonality of spawning, and (2) minimum recruitment size at first reproduction. With these results, we expect to provide data that will support the development of proposals to address lionfish management in the Baconao Biosphere Reserve.
MATERIALS AND METHODS
Study area. The selection of the study area followed criteria based on the monitoring protocol for the study of lionfish in Cuba (Acuario Nacional de Cuba, 2011). A site encompassing 10 km of coastline in the eastern sector of the Baconao Biosphere Reserve was selected (Fig. 1), including the Siboney-Juticí Ecological Reserve, one of the five core conservation areas of this biosphere reserve. As sampling biotope (1 km scale), spur and groove sites of coral reef were selected at a 0.1 km scale, considering access to the coast.

Figure 1 Study area in the Baconao Biosphere Reserve, south-eastern coast of Cuba. 1. Aguadores Este; 2. Sardinero Oeste; 3. Sardinero Este; 4. El Mangle; 5. Juticí Oeste; 6. Juticí Este; 7. Caballo Blanco; 8. La Cantera; 9. Playa Siboney; 10. Bucanero; 11. Playa Juraguá; 12. Playa Damajayabo.
This area is characterized by a coast of tectonic origin, with no platform, but with abundant submarine terraces, where coral reef grows at short distance from the coastline (100-200 m in some areas). The surface sea current runs from east-to-west with a maximum speed of 50 cm.s-1. Salinity is stable, with values slightly above 36 ppt (lonin et al., 1977). Sea surface temperature fluctuates around 27.6°C across the entire area (García, 1989).
Capture method. Sea lionfish catches were performed on a monthly basis, from April 2012 until May 2013; the only exception was November 2012 because of the impact of Hurricane Sandy in Santiago de Cuba. Catches were carried out with scuba-diving equipment at 15 to 30 m depth by dive computer Mares Puck Pro®. Fish were collected by speargun fishing, and all the animals sighted during diving were caught, regardless of fish size. On the surface, fish were placed in a cooler chest and transported to the laboratory at the Siboney-Juticí Ecological Reserve, where they were kept refrigerated and processed within 24 hours.
Data collection. Total length (LT, cm) of each fish was measured with a board to the nearest 1 cm. Total weight (MT, g) was recorded with a dynamometer to the nearest 1 g (± 1 g weight error). Then, fish were dissected by following the procedures detailed in Green et al. (2012b). The Kruskal-Wallis test was run to test for differences between monthly medians of both total length and total weight. The significance level applied was α = 0.05. The weight of gonads, liver, and fat were recorded with an analytical balance (to the nearest 0.001 g). The U Mann-Whitney test was used to explore the statistical significance of differences in size between males and females. A Chi-square test (α = 0.05) was used to determine significant differences between months.
Data processing to determine the breeding season. To determine the breeding season, the gonadosomatic index was calculated using the following equation:
where:
IG |
= Gonadosomatic index. |
MG |
= Weight of both gonads (g). |
MT |
= Total weight without stomach content (g). |
Mean monthly IG values were plotted to determine the peak of spawning, when this index decrease in an annual cycle.
The hepatosomatic index (IH) was used as a quantification of cyclical changes in accumulation of reserves.
Additionally, a body fat index was determined, as this is a reserve substance that accumulates before the onset of breeding; the following formula was applied:
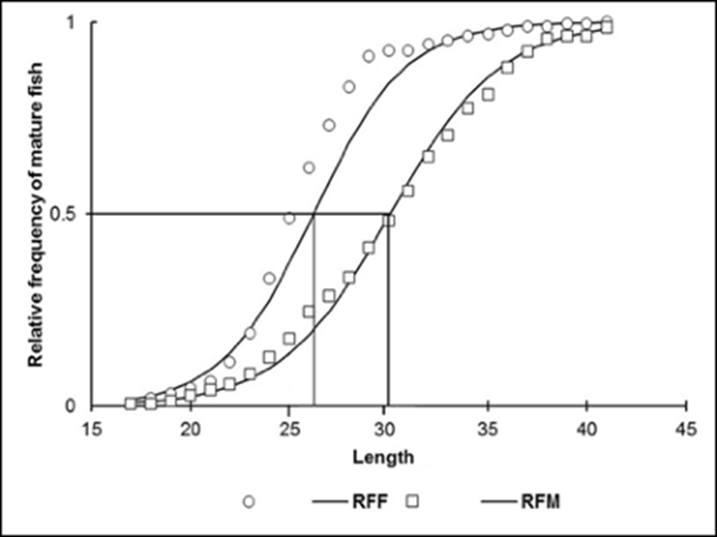
Data processing of average size at maturity. Organisms in the maturity, spawning and post-spawning stages (It was determined based in GDS III, IV, V, and VI according to Morris et al. (2011) and Priyadharsini et al. (2013), regardless of sex) were used to estimate the size at first maturity (L0.5) defined as the length at which 50% of organisms are sexually mature. Results were plotted and fitted to a logistic function not linear (Gaertner & Laloe, 1986; Sparre & Venema, 1997).
RESULTS
We caught a total of 535 individuals with an average size of 26.8 (standard deviation SD = 2.1 cm). The mean total weight was 290.6 g (SD = 197.6). The largest and smallest fish caught were 42.2 cm and 13.8 cm, respectively. In general, individuals over 35 cm were found at depths greater than 30 m, while fish under 10 cm were observed at depths of less than 10 m. To note, the latter were not collected.
The Kruskal-Wallis test showed significant differences between the monthly medians of total length (K-W = 58913, p< 0.001) and total weight (K-W = 53936, p< 0.001). Females had a mean total length of 24.7 cm (SD = 3.9) and a mean weight of 207.8 g (SD = 96.1); males reached a mean length of 29.5 cm (SD = 6.1) and a mean weight of 382.1 g (SD = 231.3). The largest fish caught was a 42.3 cm long male weighing 1600 g, while the smallest (undifferentiated) measured 13.8 cm and weighted 10 g.
The microscopic examination of all fish caught showed that 218 fish were females and 233 were males; however, sex could not be determined in 84 fish (Fig. 2). In the remaining specimens, gonads were either damaged or immature, thus precluding the identification of the sex. The Chi-square test (X2 = 0.499, p = 0.480) showed a 1:1 (F:M) sex ratio; only in May 2012, when 27 males and 13 females were sampled, there was a significant difference (X2 = 4.225, p = 0.03983).

Figure 2 Number of lionfish captured during the sampling period. Baconao Biosphere Reserve, south-eastern coast of Cuba. April 2012 to May 2013.
The size structure by sex revealed that females were mostly in the 22-31 cm size range, while males dominate from 31 cm onward (Fig. 3). In this regard, significant differences in size between males and females were found (U Mann-Whitney: U = 18161.00 [LT], U =13157.50 [MT]; p< 0.001).
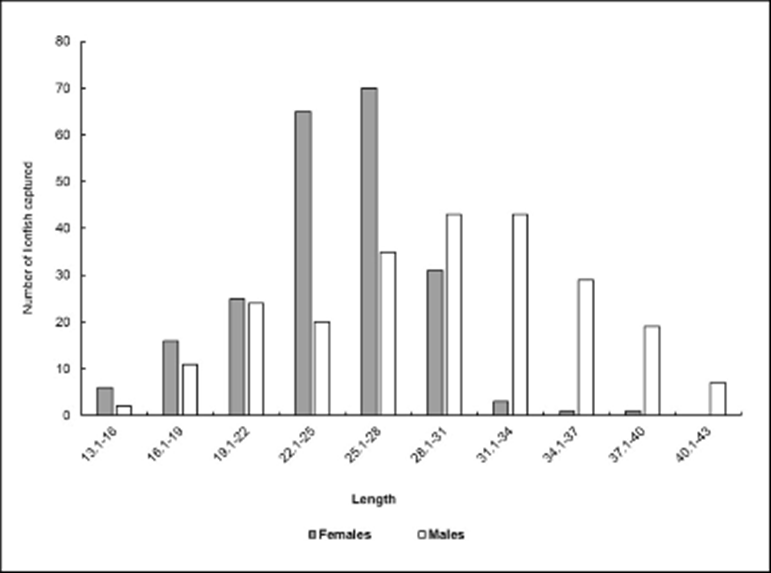
Figure 3 Distribution of sexes by size class. Baconao Biosphere Reserve, south-eastern coast of Cuba. April 2012 to May 2013.
Breeding season. The female GI values show a small decrease from April to June 2012 (4.5 to 2.8) and from March to May 2013 (5.6 to 3.9), which indicates reproductive activity. Males and undifferentiated were not considered since they did not show significant variation (Fig 4 A). Opposite trends were observed between the hepatosomatic index (IH) and the body fat index (IF) (Figs. 4 B y C), as well as between each of these two indices with IG, during spring and summer; also, significant differences were observed in this indices throughout the study period (X2 (IH)= 82.693, p< 0.001; X2 (IF)= 54.818, p< 0.001).
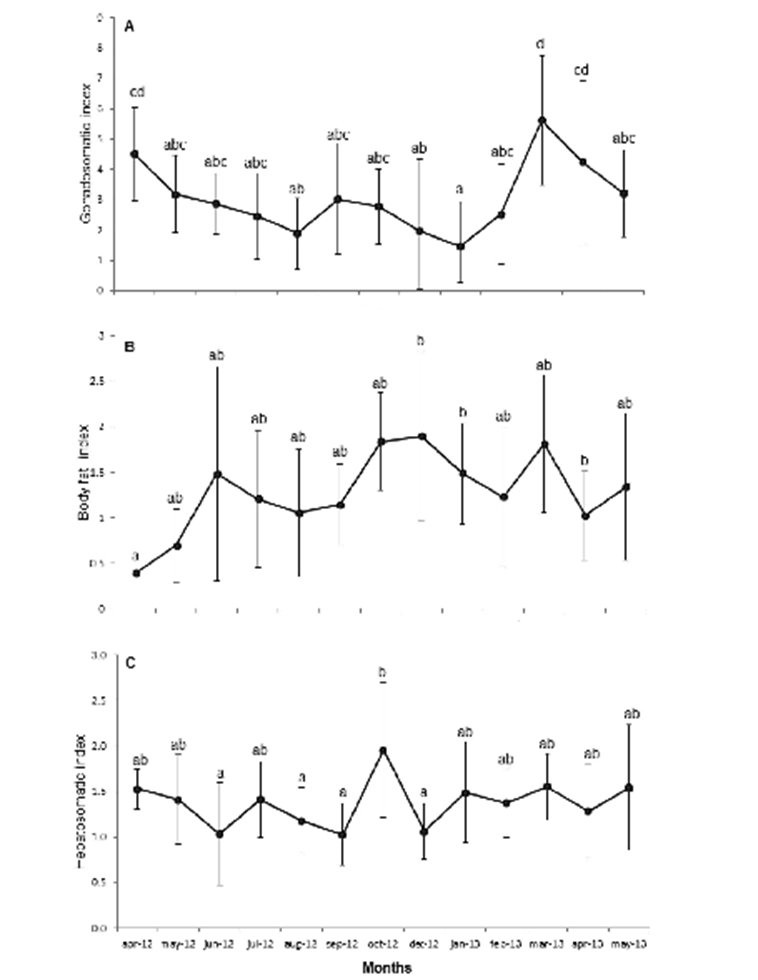
Figure 4 Monthly variation of mean values A) Gonadosomatic index (IG); B) Body fat index (IF), C); Hepatosomatic index (IH), of the lionfish population sampled in Baconao Biosphere Reserve, south-eastern coast of Cuba. April 2012 to May 2013.
Recruitment size at reproduction. The size at first maturity of females was 26.2 cm (a= 11.406; b=0.4352); for males, it was 30.07 cm (a=10.94; b=0.36). The smallest mature female was 17.5 cm; the smallest male, 17.9 cm (Fig. 5).
DISCUSSION
Mean total length of the fish caught during this study is similar to the mean value reported in the reefs of Santa Marta, Colombia (24.8 cm; González et al., 2011). On the other hand, Froese and Pauly (2018) reported a maximum length of 35 cm, which is lower than the length of the largest fish caught in this study (42.2 cm), and similar to the maximum length reported by Baker et al. (2004) in North Carolina (43 cm). The observations in this work highlight the presence of small fish at shallow depths and larger fish at deeper depths, suggesting segregation according to size across the water column, which opens up a new line of research for future studies. Similarly, the predominance of females in sizes between 15-28 cm at a depth around 25 m suggests a possible bathymetric segregation by sex, as males >30 cm in length prevail beyond 30 m depth, as observed in Turks and Caicos Islands, Bahamas, and in Roatán, Honduras (Claydon et al., 2012; Babour et al., 2010; Biggs & Olden, 2011).
Variations in IG values suggest an asynchronous reproductive cycle, which is consistent with the findings reported by Morris (2009) in waters of North Carolina, South Carolina, and the Bahamas. This reproductive profile partially explains the rapid dispersal, invasion, and colonization of lionfish in the Caribbean and Atlantic, as a result of the favorable environmental conditions (Morris, 2009; Morris et al., 2011). These include the presence of abundant food for adults, ensuring gonad development at a proper temperature (27.6 °C) (García, 1989) in the study area, known to have a positive effect on reproduction in this species. This temperature is well above the temperature limiting the development of this species, as determined by Kimball et al. (2004) (feeding stops at 16.1 °C; 10 °C is lethal). A factor closely related to temperature is photoperiod, also showing little variation in tropical latitudes; both factors contribute to constant food availability (https://searchworks.stanford.edu/view/1077428).
The peak in reproductive activity in winter was reported previously in Florida waters by Ruiz-Carus et al. (2006). When these spawning seasons is compared with reports for the natural range of lionfish in the southeast coast of India in August (Priyadharsini et al., 2013), an important difference of 4-7 months emerges, which may be an adaptive response of this species to conditions in Cuban waters.
The opposite trends of IH and IF values during the months when lower IG values were recorded may be explained by the accumulation of reserves, mainly lipids and vitellogenin, the yolk precursor stored in oocytes during vitellogenesis (Love, 1970; Van Bohemen et al., 1981). In this study, IG and IH apparently do not follow an opposite trend, with both decreasing as a result of reproduction. This is assumed to happen as a consequence of a surplus energy reserve, thus allowing lionfish not to deplete its reserves. This is supported by the fact that lionfish is iteroparous, so the liver does not play a major role, contrasting with species inhabiting in limiting environments that impose the need to store reserves to cope with starvation periods (Love, 1970; Van Bohemen et al., 1981; Saborido-Rey, 2008). To determine whether this statement is actually true would require performing bioenergetic studies. The stability of CF throughout the study period regardless of the reproductive stage reflects favorable conditions for lionfish, i.e., abundant food and absence of predators, allowing them to maintain a constant physiological performance throughout the whole year (Morris et al., 2011).
As regards the size of recruitment to the reproductive stock, the sizes at first maturity observed in the present study (26.2 cm for females and 30.07 cm for males), are larger than those by reported by Morris (2009) for North and South Carolina, Bahamas, and the Philippines, i.e., a size of first maturity of 17.5 for females and 10 cm for males in pooled samples of the three locations. The smallest female with reproductive activity reported in this study (17.5 cm) is similar to the size reported by Morris (2009) (17.2 cm), but differs from values reported by this same author for lionfish from North and South Carolina (15.8 cm) and the Bahamas (9.8 cm). In the case of males, the values reported by Morris (2009) for North (13.2 cm) and South Carolina (10.5 cm), the Bahamas and the Philippines (10 cm) differ from those recorded in the present study (17.9 cm). These differences may be explained as a response to fishing pressure by reaching maturity at smaller sizes.
The findings in this study suggest that the lionfish, P. volitans, shows an asynchronous reproductive cycle characterized by high gonadal index values during the breeding season and an inverse trend of IH and IF, suggesting that the liver and body fat store nutrients for use in reproduction.











 nova página do texto(beta)
nova página do texto(beta)