INTRODUCTION
The growing need for food at global and local level has led to the development of production methods adequately to supply the demand. Aquaculture plays an important role in this aspect and it is clear that, its role will be decisive in food production (FAO, 2016), in years to come. In Mexico, aquaculture production is a growing activity, and specifically the production of Rainbow trout has maintained a sustained growth in the last decades (García-Mondragón et al., 2013; SAGARPA, 2017). However, the production of this species begins to face associated complications related to improvement of health and nutrition of organisms in cultivation (FAO, 2018). Commercial diets for Rainbow trout don't offer any additional advantage other than the specified nutrient content, which doesn't guarantee their assimilation. This would mean food wastage, since it represents 40% to 70% of the total investment (Chao & Liao, 2007). In this sense, fish digestion is based on the production and activity of endogenous enzymes (Alarcón et al., 1997; Ray et al., 2012), and, recently, it has been reported that thatits microbiota has an important role in fish digestion, because the bacterial community wide range of hydrolases to this process (Bairagi et al., 2002; Mondal et al., 2008). Lately, in aquaculture, the development and application of probiotics has gained importance, considering that they can exert beneficial effects on the host (Ray et al., 2012; Pandiyan et al., 2013). From the physiological point of view, positive relationships have been found between probiotics and the digestion process, particularly, on the production and activity of digestive enzymes (Yanbo & Zirong, 2006; Rønnestad et al., 2013). Consequently, probiotics are an option to increasefish digestion leading to a better growth of organisms and, therefore, to aquaculture development (Zorriehzahraet al., 2016). This study aimed to determine the effect of Bacillus pumilus (BP), Bacillus sp. (BSP), and a mixture of them (BPSP) on the growth and digestive enzymes activities of Oncorhynchus mykiss, focusing on improving the production in this species.
MATERIAL AND METHODS
Bacteria. Strains Bacillus pumilus and Bacillus sp., used in the present study, were isolated, identified, and characterized as potential probiotics under in vitro conditions by Ramírez-Torrez et al. (2018).
Fish and diet. The Rainbow trout fingerlings (1.8 ± 0.03 g) were taken from the Aquaculture Center “El Zarco”, Estado de México, Mexico, and transported to the Laboratorio de Producción Acuícola at FES Iztacala-UNAM. Health condition, visible abnormalities, and absence of skin lesions or hemorrhage were corroborated on the fish. The organisms were aleatory selected and conditioned in a recirculation system for 15 days and fed with basal diet (Biofingerling of Malta Clayton®), following the feeding program indicated by the manufacturer. An average of 88 g of initial biomass was distributed in plastic tanks of 160 L each. The containers were distributed in aleatory blocks as well. Experimental groups remained independent of each other, and only same group replicates were in are circulating aquaculture system. The groups corresponded to one control (CTRL); and those treated with BP, BSP, or BPSP. For the CTRL, commercial food was provided without added bacteria. For the treatments, bacterias were added to the food at 1 × 107 CFU g-1. Each strain of the consortium had a concentration of 0.5 × 107 CFU g-1 of food. Bacteria were suspended in sterile distilled water, added to the food,mixing constantly, and aerated undera laminar flow hood for eight hours. Subsequently, the prepared food was stored according to manufacturer’s recommendations. Twice a day, fishes were fed at 6% of their biomass, as recommended by the formulated food manufacturer. Administration of bacteria in food to the experimental groups was intercalated, i.e., in the first week no group received bacteria; in the second week, the bacteria (BP, BSP, or BPSP) were added to the treated groups, except to the CTRL, and so on successively until the eighth week.
Viability of bacteria added to the food. The bacteria's viability in the food was determined as follows: a sample of 5 g of prepared food was taken, suspended in sterile saline solution (0.89%), adjusted to 10 mL, and homogenized in a culture tube. An aliquot of 100 μL was extracted, inoculated in BHI agar plates. Viability was determined by counting the CFU per gram, after an incubation of 24 h at 30 °C; this was done on the first, fourth, and seventh day of having added the bacteria to the food (Madigan et al., 2012).
From the prepared food, bacteria was recovered as follows: a homogenate was prepared, three decimal sequential dilutions were made, and aliquots were inoculated in three culture media: BHI, MRS, and TCBS (DifcoTM and BBLTM Manual, NJ, USA). Agar plates were incubated at 32 °C for 24 h. Each differentiated bacterial colony was subcultured onto culture plates and the isolated bacteria was identified by sequencing the gene encoding the 16S rRNA (Han, 2006; Mignard and Flandrois, 2006; Janda and Abbott, 2007), and amplified by the polymerase chain reaction (PCR), based onthe methodology described by Hamdan (2004) and Sambrook and Russel (2011).The DNA was isolated with the Wizard® Genomic DNA Purification Kit (PROMEGA®, Madison, WI, USA). The PCR was performed with the Master Mix® PCR kit (PROMEGA®) with a total volume of 25μL of mixture reaction, according to manufacturer’s instructions. In the thermocycler was induced (Bio-Rad® My Cycler, Hercules, CA, USA), following a pre-incubation cycle at 94°C for 5 min; 40 cycles of denaturation at 94°C for 38 s, hybridization at 52°C for 40 s, and pre extension at 72°C for 40 s; followed by an extension cycle at 72°C for 7 min and, finally, a final cooling cycle at 4°C. PCR amplicons were visualized on 1.5% agarose and stained with ethidium bromide (0.5 µgmL-1), excited under 300 nm. PCR products were purified with the Illustra® Exoprostar® kit (GE®, CT, USA); sequencing was made by Macrogen, Inc. (Seoul, South Korea) and the information obtained was analyzed using the NCBI Blast algorithm and compared with the sequences available in the GenBank data base (https://www. ncbi.nlm.nih.gov/genbank/).
The strains used had more than 90% viability.
Growth determination. Assessment of fish growth was made by measuring its biomass at the beginning, and at four and eight weeks of experimentation, and determined as:
Weight gain (WG):
Relative growth (RG; Busacker et al., 1990):
Specific growth rate (SGR; Ricker, 1979):
Where:
ln wi |
- natural logarithm of initial weight |
ln wf |
- natural logarithm of final weight |
days |
- time of experimentation in days |
Feed conversion ratio (FCR; Ramos et al., 2017):
Survival (S; Uribe & Luna-Figueroa, 2003):
Digestive somatic index (DSI; Hidalgo et al., 1999):
Enzyme activity determination. Fishes were fasted for 36 hours and, then, euthanized by thermal shock and subsequent decapitation (AVMA, 2013), and keptat 4 °C. The gastrointestinal tract (GIT) was removed and stored in an ultra-freezer (Thermo Fisher Scientific® Model: ULT1786-6-A49. Asheville, NC, USA), at -70°C, until the samples were processed. The GIT was homogenized at 2 °C and diluted 1:9 (weight: volume) in phosphate buffer (pH 7.0). The suspension was centrifuged at 22,000 g (Microfuge 22R, Beckman Coulter®, Brea, CA, USA), for 30 min at 4 °C and the supernatant was recovered. From this, aliquots of 100 and 200 μL were prepared and stored at -70 °C, until their analysis was made (AVMA, 2013; Cahu & Zambonino-Infante, 1994).
The total protein content was measured, whit the Lowry method (Waterborg, 2002), done with the Total Protein Kit, Micro Lowry, Peterson’s Modification with precipitation (Sigma-Aldrich, St. Louis, MI, USA), following the manufacturer’s specifications. The assays were designed to obtain the specific activity from crude extracts of the fish GIT (Cahu & Zambonino-Infante 1994; García-Ortega et al., 2003). To determine the specific activity of enzymes, a discontinuous spectrophotometric method was used (Nelson & Cox, 2008; Bisen, 2014; Cornish-Bowden, 2014). The generation of the product in a given period was measured, expressed as the change of the mixture in optical density (OD) (Copeland, 2000; Mantle & Harris, 2000).
Proteases. Total proteolytic activity was determined by the method of casein hydrolysis described by Kunitz (1947) and modified by Walter (1984). The total volume of the reaction was1.2 mL, composed of 250μL of 1% casein (w/v) in sterile distilled water, 250 μL of phosphate buffer, pH 7.0, and 100 μL of GIT extract; then incubated at 37 °C for 1 h. The reaction was stopped by adding 8% trichloroacetic acid (Hidalgo et al., 1999; Furné et al., 2005). The mixture was centrifuged at 1800 g for 10 min and OD was measured at 280 nm. Tyrosine was used as a standard and one unit of proteolytic activity was defined as the amount of enzyme that releases 1 μmol of tyrosine mL-1 min-1(Furné et al., 2005; Al-Saraji& Nasir, 2013; Ahumada-Hernández et al., 2014).
Lipase. Specific activity of lipase was determined by titration of fat ty acids released from the hydrolysis of an olive oil emulsion, pH 8.0 (Näher, 1974; Ali et al., 2010). The mixture’s total volume was 7.6 mL, which consisted of 2 mL of olive oil emulsion (10% of olive oil 10% of acaciagum in distilled water), 0.4 mL of 0.6%CaCl2, 1 mL of phosphate buffer (pH 7.0), 0.2 mL of crude extract, and 4 mL of absolute alcohol-acetone (1:1) phenolphthalein (0.09%). The mixture was titrated with 0.1 N NaOH. A unit of specific activity was considered as the amount of enzyme that releases 1 mmol of fatty acids in 1 min under specified conditions.
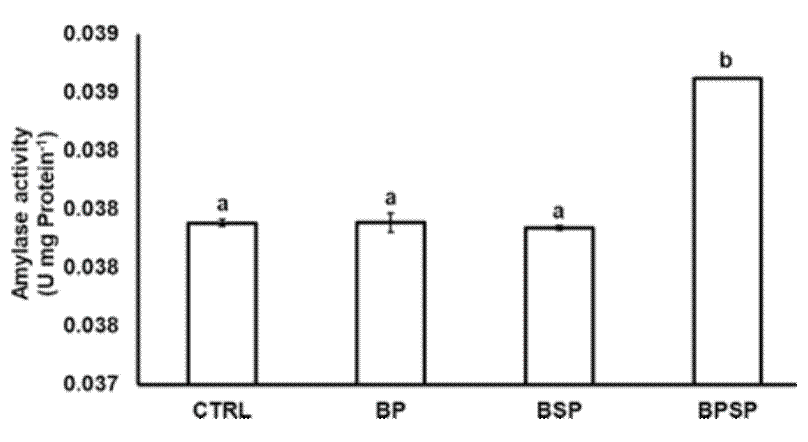
α-Amylase. Amylase activity was measured by the method described by Rick and Stegbauer (1974), based on starch hydrolysis, pH 7.0, detected from the release of the reducing group. The mixture’stotal volume was 1.2 mL, composed of 50 μL of starch solution (0.5 g of soluble starch and 17.5 mg of NaCl in 50 mL of 0.1 M potassium phosphate at pH 7.0), 50 μL of crude extract, 100 μL of dinitrosalicylate reagent (DNS, 1 g in 20 mL of 2 N NaOH and 50 ml H2O, 30 g of K-Na tartrate and 100 mL distilled water), and 1 mL of distilled water. After adding the extract, the sample was incubated at 25 °C for 5 min and after DNS addition, the mixture was incubated at 100 °C for 10 min, then the OD was measured at 546 nm. One unit was defined as the amount of enzyme that released 1 μmol of reducing groups, calculated as maltose per minute from starch hydrolysis at 25 °C, pH 7.0, and maltose as standard.
Data analysis. Biometrical data of fish and specific enzyme activities were tested for parametric statistical assumptions and one-way ANOVA was used to identify differences amongexperimental groups, considering bacterial strains as variation source. When ANOVA indicated significant differences (α <0.05), a Tukey test was applied to identify differences amonggroups (Montgomery, 2001; Zar, 2010). All analyses wereperformed with SYSTAT® ver. 12 for Windows®.
RESULTS
Growth. There was no significant difference (P> 0.05) in the initial biomass amongthe experimental units. The animals grew more than 900% after 8 weeks of experimentation; with a significant increase (P< 0.05) with respect to theirinitial weight. The survival rates among the groups didn't show significant differences. No significant effects (P> 0.05) or better growth were observed in the weight gain of the fish fed with bacteria compared to the CTRL during the eight weeks. However, when an ANOVA was made only amonggroupstreated with probiotics, the BSP group exhibited a better weight gain (15.5 ± 0.06) and was statistically different (P< 0.05), contrasted to BP (14.7 ± 0.06) and BPSP (14.1 ± 0.04). Nevertheless, no significant effects were detected in the remaining calculated parameters (Table 1).
Table 1 Growth performance of rainbow trout fed with different autochthonous probiotics. The values are the means of three replicates ± standard error. Means with different letters in the same line differ significantly (P< 0.05).
| CTRL | BP | BSP | BPSP | |
| Fish weight Ini1 - Fin2(g) | 1.8 - 17.9 | 1.8 - 16.5 | 1.8 - 17.3 | 1.7 - 15.8 |
| Change (%) | 994 | 917 | 961 | 878 |
| WG3 (g) | 16.1 ± 0.8a | 14.7 ± 0.06b | 15.5 ± 0.06a | 14.1 ± 0.04b |
| RG4 (%) | 878.5 ± 10.01 | 825.2 ± 4.81 | 862.4 ± 3.88 | 800.8 ± 1.52 |
| SGR5 (%/day) | 4.06 ± 0.01 | 3.97 ± 0.009 | 4.04 ± 0.007 | 3.92 ± 0.003 |
| FCR6 | 2.1 ± 0.1 | 2.4 ± 0.1 | 2.2 ± 0.1 | 2.5 ± 0.04 |
| DSI7 (%) | 15.03 ± 1.17 | 17.7 ± 0.97 | 19.25 ± 1.07 | 18.68 ± 0.54 |
| Survival (%) | 99.16 ± 0.8 | 95.83 ± 1.7 | 96.6 ± 0.8 | 96.6 ± 0.8 |
1Ini: initial
2Fin: final
3Weight gain
4Relative growth
5Specific growth rate
6Feed conversion rate
7Digestive somatic index
Enzyme activity. The inclusion of bacterial strains had a positive effect on the activity of the digestive enzymes. The proteolytic activity after 8 weeks of experimentation had a significant (P< 0.05) increase in fish fed with BPSP (0.23886 ± 0.000316), with the highest value, followed by BSP (0.23816 ± 0.001279), BP (0.23099 ± 0.000597), and CTRL (0.22452 ± 0.000492; Fig. 1). The lipase activity also displayed significant differences (P< 0.05) among groups and was higher in BSP (0.68284 ± 0.0001720) and BPSP (0.69012 ± 0.0004517), as compared to BP (0.68284 ± 0.0001720) and CTRL (0.68277 ± 0.0001394; Fig. 2). The amylolytic activity was higher and statistically different (P< 0.05) in BPSP (0.03877 ± 0.00000) than BS (0.03801 ± 0.00001), BP (0.03803 ± 0.00005), and CTRL (0.03803 ± 0.00002; Fig. 3). The strains mixture treatment showed the highest enzymatic activity produced in each case.

Figure 1 Total proteolytic activity of the gastrointestinal tract of Rainbow trout fed with autochthonous probiotics. Each column represents the means of three replicates ± standard error. Different letters indicate significant difference between the means (P< 0.05).
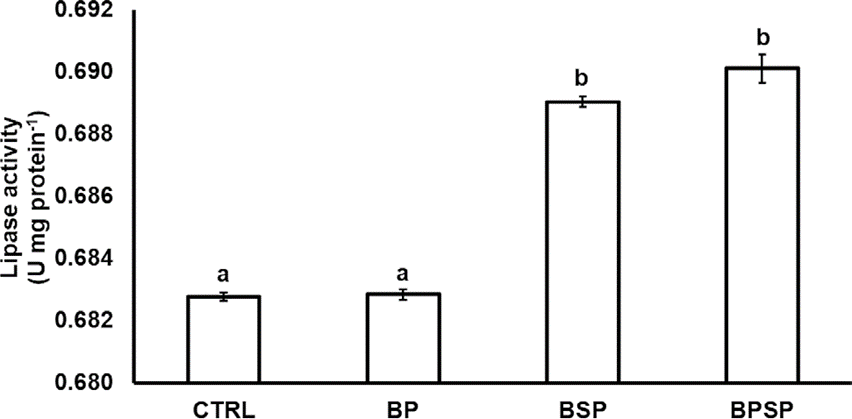
Figure 2 Lipase activity of the gastrointestinal tract of Rainbow trout fed with autochthonous probiotics. Each column represents the means of three replicates ± standard error. Different letters indicate significant difference between the means (P< 0.05).
DISCUSSION
It has been reported that Bacillus strains have a positive effect on fish growth (Austin, 2006; Gómez & Balcázar, 2008); however, they are more effective in early stages of development, probably because of the absence of a microbiota established in the GIT of the fish. On the other hand, positive results on growth have been obtained only after treating the fish with an antibiotic (Merrifield et al; 2010). In the same way, Park et al. (2017) reported that a 15-g Rainbow trout didn't show significant differences in growth. Also, it has been reported that Epinepheluscoioides (Hamilton, 1822) does not undergo a significant increase in weight gain and specific growth rate when fed with B. pumilus and B. clausii (Sun et al., 2010). In general terms, results obtained in this study agree with these authors. The density of bacteria inclusion in the food should also be considered, as Al-Saraji and Nasir (2013) reported that 1 × 105CFU g-1or 1 ×106 CFU g-1 increased significantly the growth of the common carp. However, the feeding habits of this fish should be considered, specifically when B. pumilus was used at 1 x 108 or 1 ×109 CFU g-1 of food, resulting in a significant weight gain (Srisapoome & Areechon, 2017). Probably, B. pumilus, as heterotrophic bacteria (Liu et al., 2013) exert more positive effects on fish with omnivorous feeding habits, such as Tilapia, compared to Rainbow trout, a carnivorous fish. Results indicate that there was no improvement in fish growth; however, treated fish had a homogeneous growth, which is important for aquaculture (Gisbert et al., 2014). Data point out the importance of experimentation, because from it we cank now the strain and its optimal density to obtain significant results for a specific host.
The use of probiotics in aquaculture has shown important advantages, because it can improve the digestion of fish, either by providing enzymes or stimulating secretion and, consequently, favoring the growth of organisms (Zorriehzahra et al., 2016). Bacillus species can produce a wide range of enzymes that can contribute to fish digestion (Bairagi et al., 2002); however, B. pumilus has been used most frequently to prevent and control diseases in White shrimp (Hill et al., 2009), E.coioides (Yang et al., 2014), and Nile Tilapia (Oreochromis niloticus L., 1758) (Srisapoome & Areechon, 2017), but not as a probiotic to improve digestion or growth. Monospecific probiotics have not always produced best results; in herbivorous carp, B. subtilis Ch9 did not generate benefits in enzymatic activity, during a prolonged supply (Wu et al., 2012). B. pumilus did not improve E.coioides growth, but improved the immune system response (Sun et al., 2009). On the other hand, a mixture of Bacillus spp. produced the highest activity of proteases, lipases, and amylases in common carp (Yanbo & Zirong, 2006). Nevertheless, contrary and according to Ozório et al. (2016), a bacterial consortium (B. subtilis, Enterococcus faecium, Pediococcus acidilactici, and Lactobacillus reuteri) didn't improve the growth of Rainbow trout, although anti-oxidative enzymes were lower in the treated group, but not significant against control. The results of the present study indicate that BPSP generated higher enzymatic activity, but not better growth.
Most published works have found a direct relationship between growth and activity of digestive enzymes in fish and crustaceans (Ziaei-Nejad et al., 2006; Zokaeifa et al., 2012; Hauville et al., 2016), in contrast to the results in this study in which the treatment with the mixture of strains did not show higher growth, although it produced higher enzymatic activity. When Fenneropenaeus indicus was fed with a consortium of Bacillus spp., digestive enzymes had greater activity and higher weight gain (Ziaei-Nejad et al., 2006). In Rainbow trout fingerlings an opposite behavior was observed because fish fed with B. cereus var. toyoi grew better than the control, but there was no significant effect on digestive enzymes activity (Gisbert et al., 2014). Another study showed that the use of Bacillus sp. didn't improve digestive enzymes activity or increase the growth of fish (Koca et al., 2015), in agreement with this study.
There are aspects related to probiotics that should be considered: strain and density, time and frequency of administration and fish developmental stage. Also, whether testing was made under laboratory or in culture conditions (Welker & Lim, 2011; Cha et al., 2013). The ability of B. pumilus to control a bacterial infection in Nile Tilapia has been evaluated and, at 1 × 106 CFU, fish didn't develop the infection (Aly et al., 2008). In another study, a density of 1 × 108 CFU of B. pumilus didn't show any significant effect on E. coioides growth, but there was a significant difference with B. clausii, at the same density (Sun et al., 2010). B pumilus was assessed at 1 × 1010 CFU and produced best survival rate, but not better growth, when fishes were challenged with Streptococcus iniae; it was B. subtilisthat significantly increased these two parameters (Cha et al., 2013). B. pumilus has been tested to prevent or control diseases, rather than growth (Avella et al., 2010). Its action has even been identified on genes expression associated with the immune system in the intestinal mucosa of E. coioides (Yang et al., 2014). This was also reported in Rainbow trout with Enterococcus faecium (Panigrahi et al., 2007) and L. plantarum (Pérez-Sánchez et al., 2011). In some cases, density values were higher than those used in this study and produced significant effects, and in other cases, these values were lower. This provides information about the importance of this variable and of the time of evaluation of bacterial strains with probiotic potential, that is, the relationship between the observed effects and cell densities.
It's important to consider the experimental time and the frequency of bacterial supplementation in this study. Published works evaluated growth on a daily probiotic supply (Adineh et al., 2013; Buruiană et al., 2014; Chen et al., 2016), mainly through bacteria added to food, only not many added to water (Hauville et al., 2016). In some cases, feeding was done at libitum (Giannenas et al., 2015) and in others feeding rate was calculated from fish biomass. In the present study, strains supplementation was intercalated during the eight weeks. It means that on the first week no bacteria were added to food of any experimental group; on the second week, strains were added to experimental groups, but not to the CTRL and so on until the eighth week. Therefore, a comparison between two methods, continuous and discontinuous probiotic administration, is necessary, because a beneficial effect was observed. The experimental time was eight weeks, a period in which growth changes of rainbow trout can be observed (FAO, 2018; Woynarovich et al., 2011), based that the investigation initial proposal was evaluate two strains in a specific phase growth.
In contrast with other studies, which didn't obtain significant effects after 99 days of B. amyloliquefaciens supply (Reda & Selim, 2015); in another study, significant growth was obtained in fish after 30 culture days, but not up to 60 (Sun et al., 2010). In this sense, most published works tested the strains of interest in relatively short periods, from 30 to 60 days (Giannenas et al., 2015; Afrilasari et al., 2016; Adeoye et al., 2016), and in some cases only for a week (Hauville et al., 2016), evaluating only the larval phases. Therefore, results depend mainly on the growth stage in which the bacterial strain is to be evaluated.
From a physiological point of view, significant growth effects on fish and crustaceans have been reported (Bidhan et al., 2014) regarding the bacterial enzymes contribution to the host (Bidhan et al., 2014; Allameh et al., 2017). No mechanism has been proposed to explain this relationship (Welker & Lim, 2011). Growth is an event in which many variables are involved, because not all the energy assimilated from the diet is used exclusively for growth; therefore, the digestive enzymes activity isn't necessarily related to it (Lucas & Southgagte, 2012). The fish biomass is not the only way to estimate growth (Wootton, 2011), considering that energy demand exists even in the absence of growth (Nelson, 2011). In this sense, it is necessary to assess other enzymes that are linked with fish metabolism. Not many studies have made this proposal. For example, some studies evaluated glycolytic and oxidative enzymes activity in wild Atlantic cod (Gadusmorhua L.) and obtained a strong positive relationship between these and growth (Pelletier et al., 1995). In addition, they associated protein and DNA content in muscle with previously mentioned enzymes activity.
CONCLUSIONS
The probiotics and cellular density used didn't improve fish growth, resulting in a similar fish growth in all experimental treatments; this finding can be useful for aquaculture, because it could generate homogeneous lots. Bacterial mixture had a positive and significant effect on digestive enzymes activity. In this study, autochthonous bacteria of Rainbow trouts were used not under laboratory settings, but in a study conducted under culture conditions, which provides essential information on probiotic functionality, their development, and implementation.











 nueva página del texto (beta)
nueva página del texto (beta)