Introduction
In almost every aquatic habitat, we find communities of zooplankton, including Rotifera. Zooplankton are considered to be some of the most important organisms on earth due to the food supply they provide to most aquatic life (Wetzel & Likens, 2007). Rotifers are found in different gradients of salinity and in waters of varying depths. Temporal changes in the community structure of the Rotifera assemblage are related to hydrologic phases and seasonal cycles (Frutos, 1998), and species habitat preference (De Azevedo & Costa Bonecker, 2003) for tropical lakes, which are centers of biodiversity (Lewis Jr., 2000).
The hydrological regime is reported to be one of the causes that determine community structure and biodiversity over time (Magalhães et al., 2009; Costa et al., 2013). However, we ought to know more about the influence of rainfall on the community structure, considering that many studies consider only two seasons, rainy and dry or summer and winter (Sampaio & Lopes, 2000; De Carli et al., 2017).
The seasonal variations of water levels favor the occupation of different lowland habitats that remain isolated or in connectivity, according to the season of the year, thereby providing an exchange of species between river and lake. The floodplains promote the relocation of organisms to new habitats within a period that can vary from days to weeks (Arrington & Winemiller, 2006). Changes in species richness can lead to losses of zooplankton in large quantities and impacts on succession.
The zooplankton make extensive vertical excursions through the water column (Mann, 2004) and are subject to the influence of several factors such as rainfall, temperature, water oxygen availability, pH, and electrical conductivity, which act together or separately, and may cause fluctuations in the communities, where according to Bodin and Norberg (2007), individuals within the compartments can benefit from neighboring stratum resources.
Approximately 40% of tropical lakes originate from rivers (Lewis Jr., 1996). Tropical lakes are thus part of the floodplains and are specific ecosystems with frequent interactions between land and water (Junk, 1999; Arrington & Winemiller, 2006). Floodplain lakes are considered dynamic systems, influenced by rainfall, which change with the effects of fluctuations in the water level or pulses throughout the year; they are mainly seasonal. Oxbow lakes, typical environments of the floodplain (Junk & Welcomme, 1990), are major components of Amazon watersheds, which are subject to seasonal changes and become more pronounced when considering biotic factors, such as the movement of individuals, species multiplication, and predation.
In this study, we focus on testing the hypothesis that the seasonal changes in alpha diversity and abundance (standing stocks) of Rotifera, considering also limnological variables, are mainly related to rainfall patterns. We hypothesize that 1) The diversity of Rotifera in Lake Amapá is different in the various layers of the water column; 2) The community structure is affected by precipitation due to changes in the water level; 3) Different species are dominant during different seasons.
Materials and methods
Study area. Lake Amapá is located within the municipality of Rio Branco, Acre State (Fig. 1), in southwestern Amazonia (10° 2’ 36” S and 67º 50’ 24” W). The lake is about 6 km in length and is surrounded by dense tropical forest and areas disturbed by deforestation (40%); therefore, there is an input of organic matter from the forest to the lake (Keppeler & Hardy, 2004a).
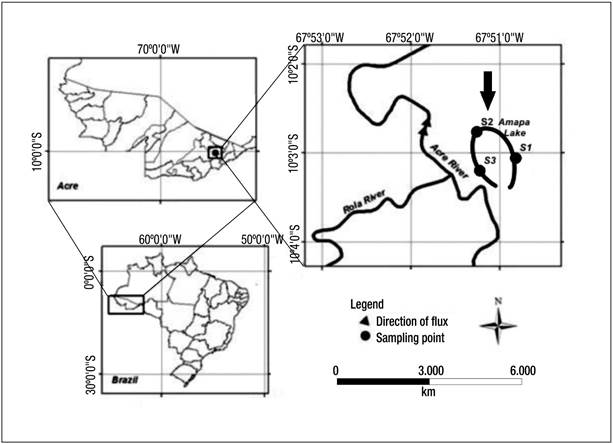
Figure 1 Location of Lake Amapá and sampling stations: S1, S2, and S3. Lake Amapá is located in Rio Branco municipality, Acre State, Amazonia, Brazil.
This oxbow lake is located at the floodplain of the Acre River, characterized as white and turbid water (Philips et al., 2008), originating in Peru at approximately 300 m.a.s.l. The river has a length of 1190 km and flows to 100 m on the right bank of the Purus River in the city of Boca Acre, Amazonas State.
Fluctuations in the water level are also characteristic of Lake Amapá, changing the hydrometric levels by the influence of hydrological periods. Figure 2 shows the three distinct phases of the hydrological cycle considered in the study.
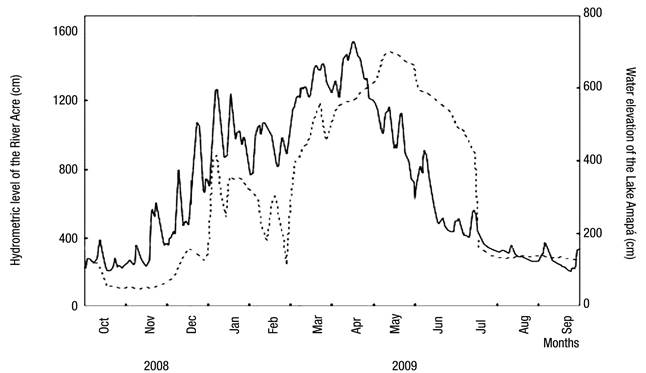
Figure 2 Daily variation in river level (solid line) and oscillation of the water level in Lake Amapá (dashed line), Rio Branco municipality, Acre State, Amazonia, Brazil, between October 2008 and September 2009. Source: Silva et al. (2013) modified.
The sub-basin of the Acre River is located in the depression of Acre River, and has predominantly sedimentary rocks of lacustrine and fluvial origin, making soils extremely poor (Fittkau et al., 1975). Their geologic positioning indicates that the region emerged during the Pliocene to Pleistocene (Araújo et al., 2005).
The fish diversity in Lake Amapá is high; between 2008 and 2009, 2,131 fish were collected, belonging to 53 species, 18 families, and five orders (Silva et al., 2013). According to Pereira et al. (2011) and Silva et al. (2013), the lake is largely predominated by small species such as Hypoptopoma gulare (Siluriformes) and medium-size species such as Triportheus curteus during the dry season.
According to the Köppen climate classification, the climate can be characterized as hot and humid (Peel et al., 2007). This is due to the Intertropical Convergence Zone in the region (Cox & Moore, 2010). Monthly data on rainfall and air temperature in the study area were obtained from the website http://sinda.crn2.inpe.br/PCD/SITE/novo/site/index.php. At this site, we found that with regard to air temperature, June (2009) and September (2009) showed the lowest and highest values, respectively, of 28.4 ºC and 29.5 ºC.
Hydrologic Regime. In this study, we define a model based on rainfall with four seasons: very rainy, rainy, dry, and very dry. The model was divided into four seasons throughout the year based on these values: Very rainy (January, February, March, April); Dry (May, June, July); Very dry (August, September), Rainy (October, November, December).
Zooplankton. Qualitative zooplankton sampling was conducted between October 2008 and September 2009 at three sampling stations in the pelagic region of the lake. Quantitative zooplankton samples were obtained between November 2008 and September 2009, covering the four seasons. Rotifera were collected with a submerged motorized pump, such that 200 liters of water were filtered in the water column using a plankton net with a 55-µm mesh for qualitative analysis, amounting to 36 samples. Quantitative samplings were also carried out at three vertical strata, with a Van Dorn bottle: surface, middle, and bottom, totaling 103 samples. The samples at the water surface were collected from the water column at the surface (S) just 0.15 m below the surface layer, in the middle (M) at the midpoint of the water column, and at the bottom (B), which we considered to be 0.20 m above the sediment (Passarinho et al., 2013).
Samples for zooplankton quantitative and qualitative analyses were stored in 300 ml glass vials, fixed, and preserved with 4% formaldehyde. Species richness of the zooplankton community was determined using a light microscope and specialized literature, such as Koste (1978), Koste & Hardy (1984), and Segers (1995). The quantitative zooplankton was estimated following recommendations in Wetzel & Likens (1991), who report analyses made with a Sedgwick-Rafter counting chamber (1 mL) under a light microscope. A minimum of 80 individuals (Bottrell et al., 1976) of the most abundant taxa were counted. The entire sample was inspected for rare species. Quantitative final enumeration has been expressed using ind. L-1.
The frequency of occurrence (Fo) of the species was calculated considering the ratio of the number of samples in which the organism was identified and the total number of samples collected, in accordance with Mateucci and Colma (1982). The following classification categories were considered: very common ≥ 70%, frequent >40% and < 70%, infrequent >10%n and <40%, sporadic or rare < 10%.
Between November 2008 and September 2009, we conducted monthly physical and chemical analyses at three stations. Limnological variables were measured: Transparency (Secchi disc in cm), depth temperature, pH, and electrical conductivity with a YSI limnological probe, while also considering the limnological variables at the surface, middle, and bottom. These variables were used to compare the Rotifera with the seasons.
Species Richness of Rotifera. The degree of significance of all values for the Rotifera diversity in four seasons was calculated with an analysis of variance (ANOVA), followed by Tukey’s post hoc Test. We used Multiple Linear Regression Analysis (r2) for the analyses of correlation between the rainfall and the diversity of Rotifera variables at each station. All these values were also calculated utilizing the Past statistics package, version 3.x. (Hammer et al., 2001).
Species Diversity and Abundance of Rotifera. The Menhinick index (Menhinick, 1964) was calculated, considering the relative proportion of a particular species in the sample. The specific diversity was estimated by the Shannon-Wiener index (H’). The values for the Shannon-Wiener index are between 0 and 1; values >0.5 indicate a more uniform proportion of the individuals among the species (Ludwig & Renolds, 1988). The equability J index (Pielou, 1966) considers the relative proportion of this species in the samples. The Simpson index is a measure of evenness (Magurran, 2004). All these values were calculated utilizing the Past statistics package, version 3.x. (Hammer et al., 2001).
Using an analysis of variance (ANOVA) followed by Tukey’s post hoc Test, we calculated the degree of significance of all values for the Rotifera and limnological variables at the surface, middle, and bottom of the water column over four seasons. We also ran a nonparametric Kruskal-Wallis test followed by Dunn’s multiple comparison test when the assumptions of normality (Shapiro-Wilk) and homoscedasticity (Levene) for the ANOVAs were not achieved with a transformation of data (log10). All these values were calculated utilizing the Bioestat 5.0 (Ayres et al., 2007).
Spearman’s coefficient was used, and we adopted a 5% significance level to test the correlation between the limnological variables with the abundance of rotifers in each layer of the water column, within each season.
Results
Hydrologic Regime.Figure 3 shows the four season divisions. Between October 2008 and September 2009, the maximum and minimum values of rainfall occurred in November 2008 (118.77 mm) and September 2009 (10.15 mm), respectively.
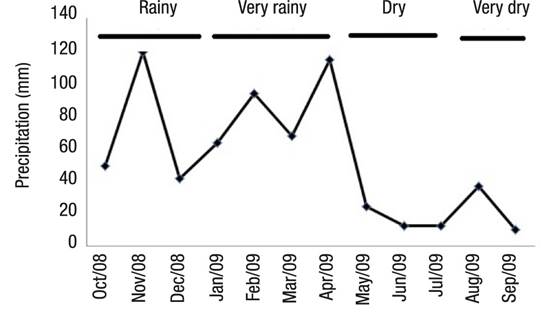
Figure 3 Precipitation at Lake Amapá (Rio Branco, Acre), between October 2008 and September 2009 in Rio Branco municipality, Acre State, Amazonia, Brazil.
Species Richness of Rotifera. Fifty-seven species of rotifers were identified. The rotifers were represented by 17 families listed in Table 1 with Brachionidae (12) and Trichocercidae (6) showing the largest number of taxa.
Table 1 Rotifera in Lake Amapá between October 2008 and September 2009. FO (%) = Occurrence frequency.
| Phylum Rotifera, Orden Rotaria | FO (%) | FO (%) | |
|---|---|---|---|
| Family Asplanchnidae | Family Gastropodidae | ||
| Asplanchna brightwelli Gosse, 1950 | 8 | Ascomorpha ovalis Bergendal, 1892 | 8 |
| Asplanchna sieboldi Leydig, 1854 | 8 | Ascomorpha sp. | 17 |
| Asplanchna sp. | 8 | Family Kerateliidae | |
| Family Brachioniidae | Keratella americana Carlin, 1943 | 58 | |
| Brachionus calyciflorus anuraeformis Brehm, 1909 | 83 | Keratella cochlearis Plate, 1886 | 58 |
| Brachionus calyciflorus Pallas, 1766 | 8 | Keratella tropica Apstein, 1907 | 25 |
| Brachionus caudatus Barrois and Daday, 1894 | 8 | Family Lecanidae | |
| Brachionus dolabratus Harring, 1914 | 58 | Lecane curvicornis Murray, 1913 | 8 |
| Brachionus falcatus Zacharias, 1898 | 33 | Lecane elsa Hauer, 1931 | 8 |
| Brachionus havanaensis Rousselet, 1911 | 83 | Lecane luna O.F. Muller, 1776 | 8 |
| Brachionus plicatilis O. F. Muller 1786 | 17 | Lecane lunaris Ehrenberg, 1832 | 8 |
| Brachionus urceolaris O. F. Muller, 1773 | 8 | Family Lepadellidae | |
| Cephalodella gibba Ehrenberg, 1938 | 8 | Lepadella ovalis O. F. Muller 1786 | 8 |
| Cephalodella sp. | 25 | Lepadella spp. | 16 |
| Notholca spp. | 74 | Family Notomatidae | |
| Paranuraeopsis sp. | 8 | Notommata sp. | 8 |
| Plationus patulus O.F. Muller, 1786 | 8 | Family Proalidae | |
| Platyias quadricornis Ehrenberg, 1832 | 8 | Proales sp. | 17 |
| Family Colurellidae | Family Synchaetidae | ||
| Colurella sp. | 8 | Polyarthra sp. | 8 |
| Family Epiphanidae | Polyarthra vulgaris Carlin, 1943 | 8 | |
| Epiphanes macrourus Barrois and Daday, 1894 | 8 | Family Testudinellidae | |
| Epiphanes sp. | 8 | Testudinella tridentata Smimoy, 1931 | 8 |
| Family Euchlanidae | Testudinella patina aspi Carlin, 1939 | 8 | |
| Euchlanis dilatata Ehrenberg, 1832 | 8 | Testudinella sp. | 8 |
| Family Filiniidae | Family Trichocercidae | ||
| Filinia longiseta Ehrenberg, 1834 | 17 | Trichocerca bicristata Gosse, 1886 | 8 |
| Filinia pejeleri Hutchinson, 1986 | 42 | Trichocerca chattoni Beuchamp, 1907 | 17 |
| Filinia opoliensis Zacharias, 1898 | 83 | Trichocerca montana, Hauer, 1956 | 8 |
| Filinia sp. | 17 | Trichocerca myersi, Hauer, 1931 | 8 |
| Filinia novaezealandiae Plate, 1886 | 58 | Trichocerca tenuior Gosse, 1886 | 25 |
| Family Flosculariidae | Trichocerca similis Wierzejski, 1893 | 67 | |
| Beuchampiella eudactylota Gosse, 1886 | 8 | ||
| Floscularia sp. | 8 |
The most frequent species found throughout the study were Brachionus calyciflorus calyciflorus, Brachionus havanaensis, and Filinia opoliensis at 83%, Trichocerca similis had a frequency of 67%, while the species Brachionus dolabratus, Filinia novaezealandiae, Keratella americana, and Keratella cochlearis registered a frequency of 58%, and Filinia pejeleri had a frequency of 42% (Table 1).
Less frequent species were Keratella tropica, Brachionus calicyflorus calicyflorus, Filinia novaezealandiae, and Trichocerca tenuior at 33%, while Brachionus plicatilis, Brachionus caudatus, Tricocerca bicristata, and Trichocerca montana registered 25%.
The following species were in low abundance with a frequency of 17%: Asplanchna brightwelli, Asplanchna sieboldi, Brachionus havanaensis, Notholca sp. 2, Notholca sp. 3, Paranuraeopsis sp., Platyias quadricornis, Cephalodella gibba, Colurella sp., Epiphanes sp., and Euchlanis dilatata.
The number of species ranged from 1 to 11 over the months (Fig. 4). There was no significant difference in the diversity of rotifers between the seasons tested (F = 1.67, p = 0.1931). There was also no significant correlation between the diversity of rotifers and rainfall at the three stations (S1: p = 0.5603, r2 = 0.16; S2: p = 0.5393, r2 = 0.13; S3 = p = 0.6032, r2 = 0.10).

Figure 4 Species richness of rotifers in Lake Amapá between October 2008 and September 2009, Rio Branco municipality, Acre State, Amazonia, Brazil. Refer also to the three collection stations.
With regard to abundance (Figs 5a-c), the highest values occurred in August 2009 (very dry season), at the surface (382 ind. L-1), middle (280 ind. L-1) and bottom (302 ind. L-1) layers, showing that in August a homogeneous pattern distribution existed in the water column, also coinciding with one of the lowest amounts of rainfall. The high Menhinick index revealed high species dominance, except in the rainy season, reaching 1.35 in the layer surface in the dry season (Fig. 6b).
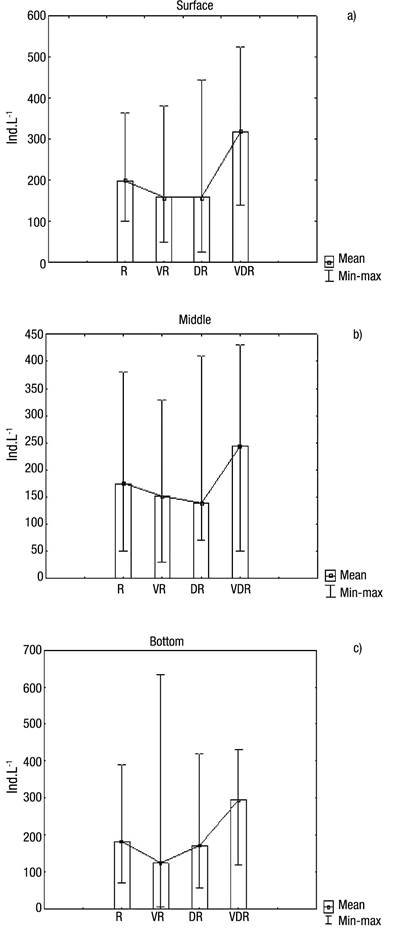
Figures 5a-c Abundance of Rotifera at the surface (a), middle (b), and bottom (c) of the water column during four seasons in Rio Branco municipality, Acre State, Amazonia, Brazil. R = Rainy; VR = Very rainy; DR = Dry; VDR = Very dry.
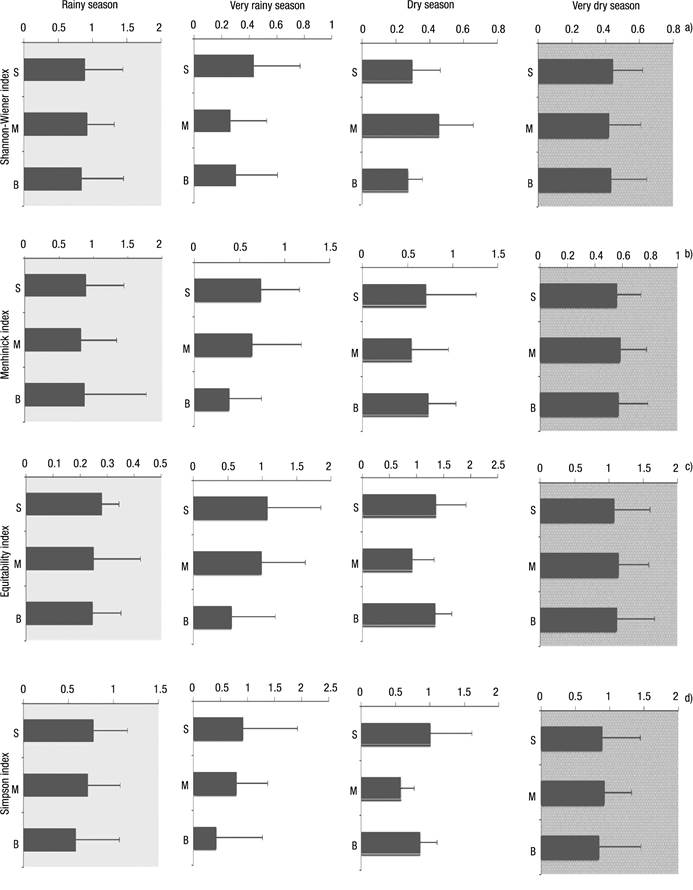
Figures 6a-d Diversity index of rotifers in Lake Amapá, between November 2008 and September 2009, in Rio Branco municipality, Acre State, Amazonia, Brazil. a) Shannon-Wiener index; b) Menhinick index; c) Equitability index ; d) Simpson index.
The Shannon index did not show a greater distribution of organisms (0.3844 to 0.8886) independent of the layer (Fig. 6a) and time season. The lower values occurred in the very dry season (Surface = 0.5579; Middle = 0.5825; Bottom = 0.5703).
In general, the equitability index showed that all species were equally abundant in all seasons, with higher values at the surface and in the middle of the water column (Fig. 6c).
Significative differences for the Rotifera were found between the rainy and dry seasons, but only at the bottom (Table 2). The limnological variables also showed differences between all the seasons in at least a layer of the water column (Table 2).
Table 2 Results of one-way ANOVAs, Kruskall Wallis* and Post hoc (Tukey and Dunn*) applied to Rotifera and limnologic variables of the Amapá Lake during seasonal periods between November 2008 and September 2009.
| F or H´ | p | Tukey or Dunn | ||||
|---|---|---|---|---|---|---|
| Rainy | Very rainy | Dry | Very dry | |||
| Abundance of Rotifera | ||||||
| Surface | 2.71 | 0.0621 | ||||
| Middle | 0.63 | 0.6024 | ||||
| Bottom* | 3.456 | 0.0296 | AB | A | AB | B |
| Limnological variables | ||||||
| Temperature | ||||||
| Surface* | 16.51 | < 0.0001 | A | B | C | AB |
| Middle* | 13.58 | < 0.0001 | A | B | BC | AD |
| Bottom* | 14.68 | 0.0021 | A | AB | AB | AC |
| Dissolved oxygen (%) | ||||||
| Surface | 1.82 | 0.1658 | ||||
| Middle | 0.1031 | 0.9568 | ||||
| Bottom | 14.68 | 0.0021 | A | A | A | AB |
| Dissolved oxygen (mg.L-1) | ||||||
| Surface | 2.4688 | 0.0855 | ||||
| Middle* | 21.9721 | 0.0001 | A | A | B | A |
| Bottom | 4.3567 | 0.2254 | ||||
| pH | ||||||
| Surface* | 13.31 | 0.0040 | A | AB | AC | ABC |
| Middle* | 19.17 | < 0.0001 | A | A | B | C |
| Bottom* | 14.93 | 0.0019 | A | A | A | AB |
| Electrical conductivity | ||||||
| Surface | 28.35 | < 0.0001 | A | B | BC | BCD |
| Middle* | 21.97 | < 0.0001 | A | A | B | A |
| Bottom* | 20.13 | <0.0001 | A | BD | CD | D |
| Transparency | 6.9431 | 0.0737 | ||||
| Depth* | 6.73 | 0.0017 | A | B | A | AC |
In the dry season, correlation between Rotifera and pH was significant (r S = 0.8000; p <0.05) in the middle of the water column and, also in this layer, between Rotifera and dissolved oxygen (%) (r S = 0.7166; p <0.05) . In the very rainy season, the dissolved oxygen also showed a significant correlation with bottom-dwelling rotifers (rS = 0.7619; p = 0.7619), and, during the very dry season at the bottom, there was also a significant correlation between Rotifera and dissolved oxygen (r S = 0.9000; p <0.05).
At the surface in the rainy season, there were several peaks, especially for Brachionus falcatus (110 ind. L-1) in November 2008 at S1 and Filinia opoliensis (180 ind. L-1) in January 2009 at S2. At the end of the very dry season, Filinia opoliensis reached a peak of 210 ind. L-1 in September 2009 at S2, followed by Brachionus falcatus (140 ind. L-1) in August 2009 at S1.
Filinia opoliensis was highest with 180 ind. L-1 followed by Filinia pejeleri (120 ind. L-1), both in November 2009 at S2. Finally, Brachionus falcatus registered 105 ind. L-1 in November at S1 in the rainy season. In the dry season, Filinia opoliensis also exhibited a major peak between 170 ind. L-1 in July at S3 and 210 ind. L-1 in September at S2 followed by Brachionus falcatus with 85 ind. L-1 (August 2009) at S1. The Simpson index reached 1 in the dry season, just at the surface, since this stratum is considered to have higher diversity (Fig. 6d).
The highest peaks were for Filinia opoliensis (290 ind. L-1) in September 2009 in S3 and Brachionus falcatus (190 ind. L-1) in August 2009, both of which occurred in the dry season.
The other organisms had sporadic abundance in the lake and at any specific station throughout the study period. Brachionus calyciflorus showed peaks in November and January 2009 (rainy season and very rainy season), at station S1 (100 ind. L-1). Brachionus havanaensis showed peaks in January 2009 at S1, respectively 50, 55, and 40 ind. L-1 at the surface, middle, and bottom layers. Keratella americana showed peaks of 65 ind. L-1 and 104 ind. L-1 at the surface and throughout the water column in July 2009 (dry season). There was a peak for Trichocerca tenuior of 65 ind. L-1 at the middle of the water column (S1) in September 2009 (very dry season).
Limnological Variables. In general, the variables showed little change, except the variables of depth, transparency, and dissolved oxygen (Figs 7a-g). The only difference occurred with electric conductivity, which showed significant differences between the dry and rainy seasons (Table 2).
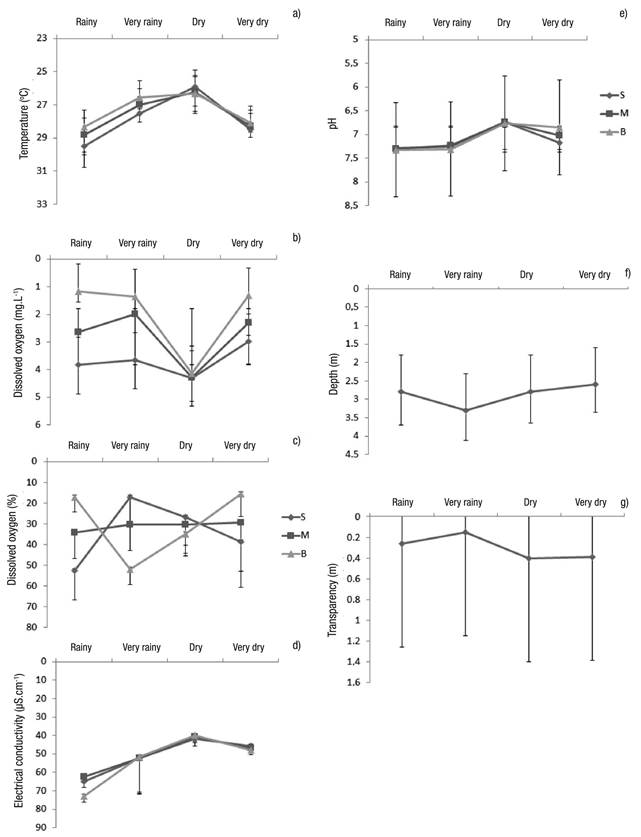
Figures 7a-g Limnological variables (mean of the seasons considering the three sampling stations ± standard error at Lake Amapá between November 2009 and September 2008) in Rio Branco municipality, Acre State, Amazonia, Brazil. a) temperature (oC); b) Dissolved oxygen (mg.L-1); c) Dissolved oxygen (%); d) Electrical conductivity (µS.cm-1); e) pH; f) Depth (m); g) Transparency (m).
Discussion
The distribution of rainfall is a limiting factor in aquatic ecosystems (Junk, 1999). It defines the seasonal cycle and directly influences ecosystem dynamics, because the rainfall pattern is responsible for water level fluctuation, i.e., the main force driving the lives of the aquatic organisms and the water characteristics of the environments and others associated with them, mainly those situated in its alluvial plain.
Several studies on limnology in the Amazon consider only two seasonal periods (Keppeler & Hardy, 2004a,b; Martins et al., 2006; Magalhães et al., 2009; Philips et al., 2008, Costa et al., 2013; Passarinho et al., 2013), but we have observed several intensities of rainfall throughout the year. This results in different categories that are reflected in the changes in precipitation in the lake water level. Recently, in studies in Lake Amapá, Silva et al. (2013) showed that water level oscillations resulted in the following: (i) pre-flooding or rising water (between October and December 2008), in which the lake was isolated from the river; (ii) minor flood (between January and March 2009), in which the lake was permanently connected to the river and when the river level reached 1260 cm; (iii) major flood (between April and June 2009), in which the lake was permanently connected to the river and when the river level reached 1550 cm; and (iv) post-flooding (between July and September 2009), in which the lake was disconnected from the river due to the fall of water. Pereira et al. (2011) also asserted that this lake was isolated from the river during the entire dry season and connected only during the flood period. From October-December, Lake Amapá is isolated from the Acre River (Silva et al., 2013), limiting the flow of species to the environment and promoting the migration of species from river to lake or among lakes in the same floodplains, because during the rainy period, the river water enters the lake through the northeast channel.
From more than 250 species of Rotifera listed for the Amazon by Robertson & Hardy (1984), in this study we found 57 species or about 23% for the Amazonia. This number (57) was higher than that encountered by Keppeler (2003) and Keppeler & Hardy Rodrigues (2004b). Comparing our results with other studies of the Legal Amazonia and the surroundings, which cover the Cuiabá River, we find that this number was a little lower than that for Lake Souza Lima (71 species) and higher than that for Lake Parque Atalaia (17 species), both studied by Neves et al. (2003). Those are marginal lakes of the Cuiabá River, revealing that the diversity in these systems can vary greatly. The reason for higher richness is that the lakes of the flood plain are heterogeneous (Lansac-Tôha et al., 2009). This happened possibly due to rainfall and the flood that occurred during the year.
The common families in this study were Brachionidae and Trichocercidae. Silva et al. (2012), studying the small Jesumira River located within the Serra National Park of Divisor, also found these families to be the most representative. High numbers of diversity for these families have also been cited in other environments of the Amazon region: Trichocerca by Brandorff & Koste (1982), Nova et al. (2014), and Brachionus by Koste & Hardy Rodrigues (1984). Brachionidae was also the richest species according to De Paggi et al. (2012), who investigated a shallow lake in a floodplain in Argentina. These families are widely distributed in South America.
Regarding the Lecane species, although Sharma & Hatimuria (2017) report on a tropical environment, in our study the low diversity and density is perhaps due to the fact that there were not habitats as macrophytes to shelter and protect for this species in lake studied.
During the course of the year in Sul Ocidental Amazonia, the water chemistry influenced the alpha diversity and standing stock (abundance) of Rotifera (Koste & Hardy Rodrigues, 1984). As we have seen in this study, the rainfall controls the distribution of Rotifera in the lake, which negatively affects abundance with marked differences between the dry and rainy periods.
Seasonality plays a key role in the zooplankton community structure, an aspect that was studied by Martins et al. (2006) and Negreiros et al. (2010). The standing stocks of rotifers decreased in the rainy season. Rains bring nutrients capable of maintaining a high production of zooplankton in the rivers, whose surplus diversity can be exported to surrounding environments, such as the lakes.
Generally during the rainy season, there was a 33% reduction in the abundance of Rotifera. In addition, the low standing stocks observed in the very rainy season are probably due to the prevailing poor oxygen conditions and, in a context of extreme floods, the currents. The standing-stock development is low in the very rainy season and is similar to that observed in other lakes in Amazonia (Carvalho, 1983; Koste & Hardy Rodrigues, 1984).
Indirect effects, such as rainfall, which cause pulses in the lake, can explain the reverse migration of zooplankton. In general, the species were distributed homogeneously in the water column. If a lake is relatively shallow, a large proportion of the water column has enough light to support photosynthesis (Mann, 2004).
The species Brachionus falcatus, Filinia opoliensis, and Filinia pejeleri contributed most of the standing stocks. In this study, rotifers had correlation with acidity. These genera and/or species were also observed in other relatively acidic environments (pH = 5.70 to 7.11) in floodplain lakes of the Caura River, Venezuela, corroborating the results of Reverol et al. (2008).
The rotifers in the lake varied in size from 40 to 2000 μm. In experimental laboratory conditions it was observed that the population of Asplanchna increases with the availability of Brachionus in the medium (Sarma et al., 2003), and the rate of population increase (r) rises with increasing temperature to 25 oC (Pavón-Meza et al., 2005) or greater than 25 oC. Such conditions favored the growth of Asplanchna species in the study (Keppeler, 2003; Keppeler & Hardy Rodrigues, 2004a).
The values of dissolved oxygen were generally low (0.45 to 3.95 mg.L-1), especially in the rainy and very rainy period, indirectly showing low food availability. In marginal lakes, food availability is lower in the rainy season, because the phytoplankton also decrease in abundance, which is also caused by dilution, resulting from the increase in the water level. The lower residence time of the water in the lake during the rainy season also contributes to this (De Paggi & Paggi, 2007). The dissolved oxygen also showed that there may be decomposition in the lake throughout the year. This event produces toxic products that may interfere in the establishment of zooplankton populations, contributing to low diversity.
The pH was already slightly acid to neutral, independent of the seasons of our study. It is noteworthy that decomposition conditions are generally acidic. However, these values do not allow the development of plankton communities; thus, at this pH, the inorganic ions dissolved in water were possibly insufficient for the further development of the community.
In summary, the level of precipitation influences fluctuations in the communities of Rotifera at Lake Amapá and is responsible for seasonal variation. Rainfall influenced the species diversity of rotifers, expressed by various indices in this study. The diversity of Rotifera in Lake Amapá did not follow a uniform standard for different layers of the water column. In general, diversity was highest in the middle of the water column.











 text new page (beta)
text new page (beta)



