Introduction
Mysids species have a short reproductive cycle, which means they can quickly reproduce in vast numbers, and are a potentially useful food source for both wild and cultured organisms (Biju et al., 2009). In all species in the order Mysida, the females carry their embryos in the brood pouch or marsupium, a subthoracic chamber formed by pairs of overlapping oostegites, where juveniles develop until they attain the adult form (Price, 2004).
Metamysidopsis elongata (Holmes, 1900) has two currently recognized subspecies, the nominal subspecies M. e. elongata from the Pacific Ocean and M. e. atlantica (Băcescu, 1968) from the Atlantic Ocean (Băcescu, 1968).
Copulation, lasting a few seconds, occurs at night 2 or 3 minutes after the mature female has molted (Mauchline, 1980). Eggs are ejected from the external genital opening of the oviduct near the base of the sixth pair of pereiopods and the male sperm mass is placed in the pouch. The embryos (fertilized eggs) are incubated within the pouch until first larval ecdysis. The juveniles are released shortly thereafter, at night and over a short period of time, from minutes to an hour. After release, juveniles tend to sink and undergo a second larval ecdysis; statocysts appear and they are able to swim, acquiring their highly mobile juvenile form in a few minutes after release (Murano, 1999; and Mauchline, 1980).
Males and females develop distinct morphological features during the period of rapid growth before maturity (after maturity, growth becomes slower). In males, the four pleopods reach the trailing edge of the last abdominal segment, and the male lobe is fully developed and setose. Females have elongated oostegites (the marsupium growing) (Murano, 1999). However, Nair (1939) reported dimorphism in the first abdominal appendages and antennules, even though there is no development of the pouch and penis. After the release of juveniles, the female begins the molting cycle, copulation, embryos are developed, and release of juveniles again (Murano, 1999). The studies by Ortega-Salas et al. (2008) of the fecundity of Mysidopsis californica (W. M. Tattersall, 1932) from Mazatlán Bay under semi-controlled conditions showed a low correlation (r = 0.27, p = 0.196) between the number of released juveniles and female length. In addition, Rendon-Valdez (2013) studied some aspects of the reproductive biology of the mysid Amathimysis trigibba (Murano & Chess, 1987) in natural conditions in Mazatlán Bay. Calil & Borzone (2008) mentioned that tropical mysids, such as Metamysidopsis neritica (Bond-Buckup & Tavares, 1992), reproduce continually in latitudes less than 40º N. Ortega-Salas et al. (2015) studied growth and survival in M. elongate they grew. The average growth rates by the von Bertalanffy model for juvenile male and female mysids were 0.304 mm day-1, 0.149 mm day-1 and 0.208 mm day-1, respectively.
Fecundity is an important biological parameter because it indicates reproductive potential and is measured by the number of eggs, embryos, or larvae that females have in different sizes (Clutter & Theilacker, 1971; Nath, 1973; Nuñez-Lecuanda, 2013). Also, data on fecundity are important in calculating the size of a stock, so the marsupial and post-marsupial fecundity of M. elongata in Mazatlán Bay was calculated under wild conditions and in two culture generations.
Materials and methods
On Mexico’s Pacific coast in Mazatlán Bay, a surface sample of 250-300 mysids was collected manually each month from September 2010 to October 2011 over the sandy areas with a plankton net (mesh 1000 μm, mouth opening 50 cm diameter). The water temperature and salinity were recorded for each sampling date and the organisms were transferred to the laboratory. Acclimation lasted three days. Transparent bottles (4 L) were used at a seawater temperature of 22 ± 1° C, salinity 32 ± 1, photoperiod 14:10 (light: dark) provided by 40W fluorescent tubes and constant-soft aeration air-stones. Every day organisms were fed Artemia (aged 18-48 h and average length 480 μm) ad libitum; brand-INVE Aquaculture-Artemia Systems, grade A with a hatching rate of 100 000 nauplii per gram dry cyst hatched under intense fluorescent light tube at 24 °C and salinity between 33 and 35. Fifty percent of the water was replaced every two days. Males were distinguished by extended fourth pleopods, a lobe with hair-like setae, and the presence of antennae; females had elongated oostegites sufficient in size to form a pouch full size.
In 200 mysids of the wild population, marsupial stages of development were identified and described by Nuñez-Lecuanda (2013). Frequency of development stages determines the marsupial fecundity (number of embryos). A dilute menthol solution was applied to slowly numb their bodies, and after 10-15 minutes they were fixed with a solution of 4% formaldehyde for 5-10 minutes. The marsupial fecundity was determined by opening with a dissecting needle the pouch of each ovigerous female whose pouch showed no signs of damage. The terminology of Wortham-Neal and Price (2002) in Americamysis bahia (Molenock, 1969) was used for the description of embryonic stages. For the F1 and F2, the experimental design consisted of three 20 L aquaria with 100 newly released young mysids each (density of 5 ind • L-1).
The average and standard deviations of the data were processed with the Excel statistical package. Statistical analysis used StatSoftMR. Normality was tested (Kolmogorov-Smirnov with Lilliefors p <0.05) and then the nonparametric Kruskal-Wallis test was used.
To compare the proportions of ovigerous females and females with an empty pouch, a nonparametric Mann-Whitney U test was used. Significance was fixed at p <0.05. A correlation analysis between female length and number of embryos born was made.
Results
In wild female mysids (n = 1 400), the marsupium contained 6.28 ± 2.94 embryos (range 1-18). Females with 4-7 embryos accounted for 55% of the population (Fig. 1, Table 1). Average marsupial fecundity varied significantly with time (H = 259.5; p <0.05), being highest (9.7 ± 2.32) in September 2010 and lowest (4.08 ± 1.48) in August 2011 (Fig. 2). 86% of the females sampled had an average fecundity of 6.17 ± 2.84 embryos. Of the F1 females, 83% were carrying 4-6 embryos, whereas of the F2 females 96% were carrying 3-5 embryos (Fig. 3).
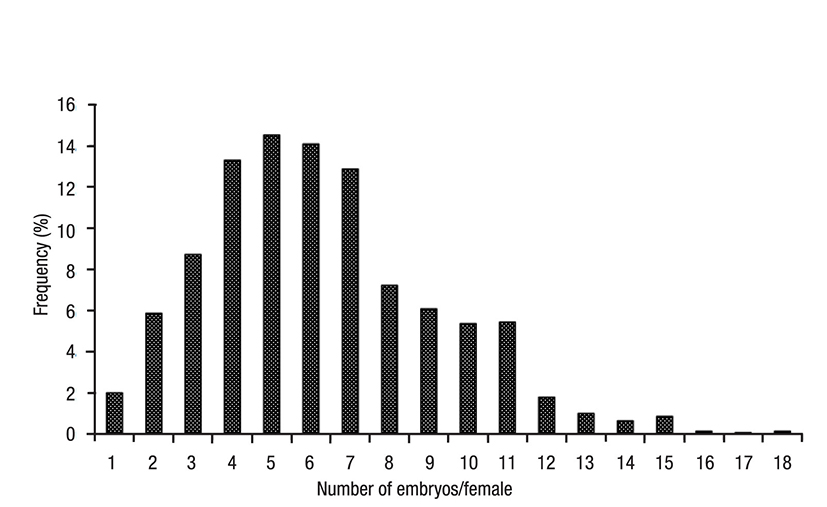
Figure 1 Frequency of fecundity in the marsupial sampling cycle of Metamysidopsis elongata (Holmes, 1900) from Mazatlán Bay, Sinaloa, Mexico.
Table 1 Embryo number (range, mean±, SD) in wild Metamysidopsis elongata (Holmes, 1900), and in cultured F1 and F2 at each development stage from Mazatlán Bay, Sinaloa, Mexico.
| Wild | F1 | F2 | |
|---|---|---|---|
| Stage I | 1-15 (5.76±2.72) (n=355) |
2-6 (4.45±1.21) (n=11) |
2-5 (3.75±0.96) (n=12) |
| Stage II | 1-18 (6.41±2.87) (n=322) |
3-6 (4.0±1.41) (n=5) |
2-5 (3.27±1.0) (n=11) |
| Stage III | 1-14 (6.34±2.74) (n=319) |
4-6 (4.5±1.0) (n=4) |
3-5 (4.0±0.7) (n=9) |
| Stage IV | 1-16 (6.31±3.18) (n=420) |
3-7 (4.81±1.25) (n=11) |
3-5 (3.91±0.79) (n=12) |

Figure 2 Marsupial fecundity of Metamysidopsis elongata (Holmes, 1900) during sampling cycle from Mazatlán Bay, Sinaloa, Mexico.

Figure 3 Frequencies of marsupial fecundities among F1 and F2 females of Metamysidopsis elongata (Holmes, 1900) from Mazatlán Bay, Sinaloa, Mexico.
Average marsupial fecundities of females F1 (4.51 ± 1.20) and F2 (3.72 ± 0.89) generations were significantly lower (H = 55.3; p <0.05, Tukey’s HSD) than in the wild females (avg. 6.28 ± 2.94), but they did not differ from each other. The wild females were significantly longer than those of the F1 and F2. Table II summarizes average fecundity ranges (SD) and the length (range, average, and SD) of wild, F1 and F2 females.
Average post-marsupial fecundity was 5.86 ± 1.45 in wild females (n = 95), 3.74 ± 1.20 in F1 females (n = 50), and 3.32 ± 1.42 in F2 females (n = 50) (Fig. 4).

Figure 4 Frequencies of post-marsupial fecundities of wild, F1, and F2 females of Metamysidopsis elongata (Holmes, 1900) from Mazatlán Bay, Sinaloa, Mexico.
The fecundity of wild post-marsupial mysids was significantly higher than that recorded in the cultures (Table 2, Fig. 4) (Nuñez-Lecuanda, 2013). The lengths of the wild, F1, and F2 females used in the evaluation of post-marsupial fecundity were not significantly different (ANOVA F1, 2,192 = 3.04, p >0.05). There was low correlation between fecundity and length in post-marsupial wild females (r = 0.05, p >0.05), and this also prevailed in F1 and F2 (r = 0.33 and r = 0.04, p >0.05 in both cases). Within each generation there was a relationship between the number of embryos and the length of the female: In the wild females, the number of embryos increased at a rate of 1.10 embryos per 1 mm increase in female length; in the F1 this increase was 0.76 embryos, and in the F2 it was 0.61.
Table 2 Length (mm), marsupial and post-marsupial fecundity of Metamysidopsis elongata (Holmes, 1900) (interval, average, SD) of wild females, F1 and F2 from Mazatlán Bay, Sinaloa, Mexico.
| Marsupial | Wild | F1 | F2 |
|---|---|---|---|
| Female length (mm) | 4.24-6.98 (5.67±0.54) |
5.28-6.52 (5.95±0.42) |
5.36-6.62 (6.08±0.37) |
| Embryos number | 1-18 (6.28±2.94) (n=1 400) |
2-7 (4.51±1.20) (n=31) |
2-5 (3.72±0.89) (n=44) |
| Post-marsupial Female length (mm) | 4.19-6.66 (5.35±0.74) (n=250) |
4.43-6.53 (5.41±0.60) (n=50) |
4.82-5.85 (5.36±0.32) (n=50 |
| Juveniles released | 3-9 (5.86±1.45) (n=95) |
1-6 (3.74±1.20) (n=50) |
3-8 (3.32±1.42) (n=50) |
| Juveniles length (mm) | 1.14-1.30 (1.24±0.03) (n=150) |
1.14-1.28 (1.20±0.03) (n=150) |
1.14-1.29 (1.20±0.04) (n=271) |
Discussion
Our observation of ovigerous females at 18-20 days was similar to the 25 days reported for M. e. atlantica (Gama et al., 2002) and (Gama et al., 2006).
Fecundity, an important part of reproductive potential, is measured by the number of eggs, embryos, and larvae with females at different sizes (Clutter & Theilacker, 1971; Nath, 1973). In general, temperate mysids produce between two to three generations per year (Mees et al., 1994), whereas in the tropics they reproduce continuously (Goodbody, 1965). This was observed in M. elongata and Acanthomysis thailandica (Murano, 1986) as well as in other tropical organisms such as Mesopodopsis orientalis (W. Tattersall, 1908) (Hanamura, 2008; Biju et al., 2009; Biju & Panampunnayil, 2010).
The temperature in the tropics where M. elongate is living is higher than other species and they reproduce continuously. During summer (22 °C), an average of 8.53 ± 0.18 embryos per female has been recorded for Mesopodopsis slabberi (Van Beneden, 1861), 42.65 ± 1.43 for Gastrosaccus spinifer (Goës, 1864), 16.04 ± 0.06 for Schistomysis kervillei (G. O. Sars, 1885), and 6.09 ± 0.35 for S. spiritus (Norman, 1860), while in M. elongata the average marsupial fecundity was 6.28 ± 2.94 embryos per female in wild mysids, 4.51 ± 1.20 in the F1 and 3.72 ± 0.89 in the F2. The average post-marsupial fecundity was 5.86±1.45 in wild mysids, 3.74 ± 1.20 in the F1, and 3.32 ± 1.42 in the F2 at temperature of 22 ± 1 °C, salinity 32 ± 1, photoperiod 14:10 (light: dark). The wild mísidos eat great variety of food, whereas the cultivated ones, only Artemia nauplii, this can be reflected in the number of offspring.
The relationship in M. elongate (6-8 mm) between the number of embryos and the lengths of wild progenitors females, F1, and F2 showed a rate of 1.10, F1 0.76, and F2 0.61 embryos for each unit of female length increase, respectively; these proportions were less than the ones found in in A. trigibba, a species of smaller size (2.76 mm) increase in length with 1.91 embryos / mm (Rendón-Valdez, 2013) and M. californica (5.56 mm) increase in length with 1.56 embryos / mm (Ortega-Salas et al., 2008). The giant mysid Gnathophausia ingens (Dohrn, 1870), the lophogastrid mysid (151 mm), has 1.0-2.31 embryos per 1 mm increase in the length of the female (Childress & Price, 1978).
Mauchline (1980) stated that mysid fecundity is directly proportional to the total length of the females, and that it is an inverse function of the metabolic rate, which is directly proportional to the water temperature. In M. elongata from Mazatlán Bay, the number of embryos was low correlated with body length of females during the sampling cycle, whether in wild-caught females, or in the F1, or F2 (r = 0.04, r = 0.005, and r = 0.04; p <0.05, respectively). In A. thailandica, the maximum number of wild embryos recorded was 18 in a female of 5.51 mm, but in other females the same size, minor and major size, the number of embryos in Stage I was 9, i.e., the same length females have different numbers of embryos (Ramarn, 2012).
The fecundity of the wild mysids was present throughout the sampling cycle and was lower than that of the cultivated mysids.
Fecundity of Metamysidopsis elongate is essential for knowing how many descendants we could have to plan our work, but also to establish the marsupial and post-marsupial fecundity in the wild and in two culturing generations (F1 and F2). The continuous fecundity in the wild mysids was present throughout the sampling cycle, although it was lower in the cultivated ones; the ones in the wild ate a variety of organisms, while the cultivated ones ate just Artemia nauplii.











 nueva página del texto (beta)
nueva página del texto (beta)


