Introduction
The addition of plant-derived feedstuffs to aquaculture dietary formulas is an alternative for producing cost-effective feeds. However, at high levels these formulas produce some side effects triggered by the presence of substances, such as mycotoxins, that may reduce the animal’s performance. Research has shown that feeding aflatoxin-contaminated diets to fish and shrimp reduces feed intake and growth performance, suppresses the immune system, and causes hepatic lesions (Arunlertaree et al., 2007; Gopinath & Raj, 2009; Mohapatra et al., 2011; Arana et al., 2011; Tapia-Salazar et al., 2012; García-Pérez et al., 2013; Zeng et al., 2015). This occurs even at low levels (Tapia-Salazar et al., 2012; García-Pérez et al., 2013; Zeng et al., 2015). In terrestrial animals, the supplementation of aluminosilicates, biotransformation agents, or antioxidant compounds neutralize the impact caused by the consumption of diets containing mycotoxins (Surai & Dvorska, 2005; Kolosova, & Stroka, 2011; Gowda et al., 2013). In aquatic organisms, particularly fish, some studies have shown that some of the negative effects caused by the presence of mycotoxins are successfully reverted by including aluminosilicates, cell walls from yeast, glucomannan, or plant extracts (Sahoo et al., 2003; El-Barabay & Mehrim 2009; Mahfouz, 2015). Con versely, other studies in which aluminosilicates were supplemented in diets that subsequently were fed to shrimp did not show promising results (Suppadit et al., 2006; Arunlertaree et al., 2007; García-Pérez et al., 2013). For some time, yeast has been used as a supplement in diets intended for consumption by terrestrial and aquatic animals in order to enhance their immune response as measured by the res pective parameters (Ringø et al., 2012; Cheng et al., 2014). Recently, it was demonstrated that supplementation of yeast-derived products (Selim et al., 2014), alone or in combination with other mycotoxin bin der/transforming products, significantly decreases mycotoxicosis in aquatic organisms (Hauptman et al., 2014). There is scarce informa tion on the effectiveness of commercially available aflatoxin binders containing multicomponent-sequestering additives with regard to re ducing aflatoxicosis in shrimp. Therefore, the aim of this study was to assess the adverse effects induced by the presence of aflatoxins on the growth rate, feed intake, feed conversion ratio, survival, and nitrogen retention efficiency. We also wanted to evaluate the potential of three commercially available anti-aflatoxin additives in preventing the nega tive effects caused by aflatoxin on a juvenile population of white shrimp Litopenaeus vannamei (Boone, 1931).
Material and methods
Experimental design: The weight of two hundred white shrimp juveniles L. vannamei, characterized by a consistent body weight (210±4 mg), were individually registered and randomly distributed in 20 groups containing ten shrimp each. Initial body weight distribution was equivalent in each group. Each dietary treatment was randomly assigned among the four tanks.
Experimental diets: Two control diets (aflatoxin-contaminated and non-contaminated diets) were formulated to contain 38% crude protein and 8% crude lipids. The aflatoxin levels included in this study were chosen based on a previous study (Tapia-Salazar et al., 2012), in which a significant decrease was observed on growth, feed intake, as well as the presence of histological damages without significantly affecting survival rates. A contaminated diet used as a control was formulated containing a total amount of 75 µg kg-1 aflatoxins (7130 µg kg-1 total aflatoxin in the contaminated grain). The NuteK S.A. de C. V. company provided contaminated corn, according to a protocol previously des cribed by Tapia-Salazar et al. (2012). The diet included 11.1% conta minated corn, mainly at the expense of wheat meal. Three more diets were prepared that included similar contamination levels and one of the following three aflatoxin binders that were tested: 1) Aflabalan® third generation (SAGARPA registration number A-7853-00). This bin der contains silica, maximum 65.60%; sodium oxide, minimum 0.20%; calcium oxide, minimum 3.90%; sodium aluminum, minimum 6.65%; and cell walls from the yeast Saccharomyces cerevisiae Meyen ex E. C. Hansen (Hansen, 1883 maximun 5% (http://www.tecnicamineral.com.mx/divisiones.php?divisionproducto=2&categoriaProducto=15). The recommended inclusion levels for use on terrestrial animals for preventive and corrective purposes range between 2 and 3 kg/ton of feed. 2) Mycosorb® (SAGARPA registration number A-7569-051). This bin der contains glucomannan cell walls from Saccharomyces cerevisiae and some varieties may contain cell walls from the microalga Chlorella vulgaris Beyerinck [Beijerinck]. (1890). https://nutricionanimal.info/mycosorb-alltech/. The recommended inclusion levels for use on te rrestrial animals range between 0.5 and 1.5 kg/ton without specifying preventive or corrective doses. 3) Mycofix plus® (SAGARPA registration number A-7160-001). This binder contains bentonite plus the yeast Trichosporon mycotoxinivorans (Molnár et al., 2004) and algae extracts. Recommended inclusion levels for terrestrial animals range between 0.5 and 1.0 for preventive dosing, whereas 1.5 - 2.5 kg/ton is recom mended for corrective doses (https://nutricionanimal.info/download/Mycofix-Plus-BBSH.pdf).
For aquatic species, no information was available on the optimal inclusion levels of aflatoxin binders for preventive and corrective dosing. Regarding Aflabalan®, a corrective level of 2 g kg-1 was used. Since there was a lack of information on terrestrial species and aquatic species, we followed the manufacturer’s recommendations. Regarding Mycofix plus®, a corrective dose of 2.5 g kg-1 was selected, given its previous positive results on terrestrial species (Tapia-Salazar et al., 2010) as per the manufacturer’s recommendations. In the case of Mycosorb®, the inclusion level was set at 2 g kg-1 because of insufficient results reported on terrestrial species at lower inclusion levels (Surai & Dvorska, 2005; Boudergue et al., 2009; Kolosova & Stroka, 2011; Gowda et al., 2013), also following the manufacturer’s recommendation (Table 1).
Table 1 Composition of all experimental diets (g kg-1as is) for Litopenaeus vannamei juveniles.
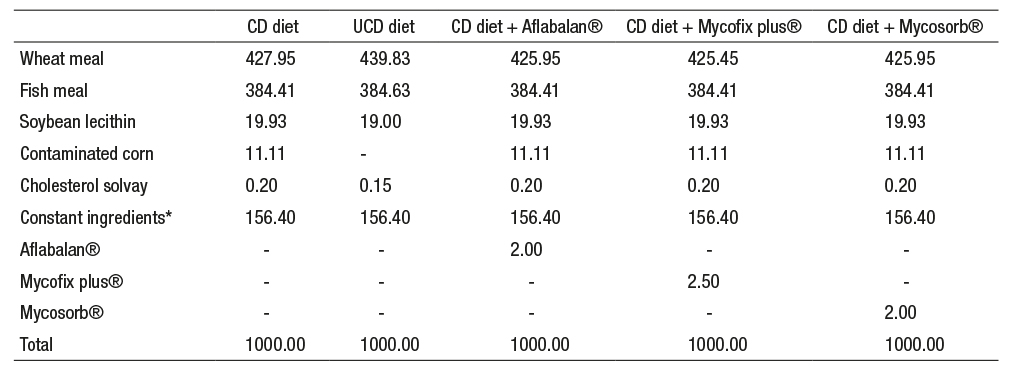
CD = contaminated diet, UCD = uncontaminated diet
*Constant ingredients: soybean meal 80 g kg-1, shrimp meal 40 g kg-1, fish oil 19 g kg-1, sodium alginate 10 g kg-1, vitamin mixture 3.5 g kg-1, mineral mixture 2.5 g kg-1, antioxidant 0.5 g kg-1, mold inhibitor 0.5 g kg-1, vitamin C 0.2 g kg-1, vitamin E 0.2 g kg-1.
Diets were prepared as follows: Ingredients were mixed for 10 min in a Kitchen Aid mixer, water (30%) was incorporated, and the mixing continued for an additional 15 min. The wet diet mash was processed through a meat grinder (fitted with a 1.6 mm diameter-hole die) at a passage rate of 40 kg min-1 while keeping the temperature between 70-75 °C. The spaghetti-like strands were placed to dry in a convection oven at 100 °C for 8 min and allowed to cool and dry overnight at room temperature before packing. The approximate composition of the experimental diets was assessed by using standard procedures (AOAC, 2006). The aflatoxin concentration (B1, B2, G1, and G2) was analyzed by HPLC on both non-contaminated and aflatoxin-contaminated con trol diets at Trilogy Analytical Laboratory (Washington, MO). Additionally, these diets were also tested for deoxynivalenol, fumonisin (B1, B2, and B3), ochratoxin A, and T-2 toxin at the same location.
Growth trial conditions and feeding protocol. A feeding trial was carried out in a closed recirculation system containing artificial seawater. The experimental facility had an array of 60-L fiberglass tanks. Each one of these was continuously fed with synthetic marine water (Fritz®, Dallas, TX, USA) using a 350 mL min-1 flow-through rate. Each tank is equipped with an air-water lift system for internal recirculation. The facility is designed to account for possible water-quality fluctuations that may simultaneously affect all tanks. Salinity and temperature were measured daily, whereas pH, total ammonia, nitrites, and nitrates were recorded weekly: salinity 31±3 g L-1, temperature 29±1 ºC, pH 8.1±0.1, total ammonia 0 mg L-1, nitrites 0.2±0.1 mg L-1, and nitrates 40±15 mg L-1 (mean ± standard deviation). During this study, these parameters were within the optimum values for shrimp. Juvenile white shrimp L. vannmaei were obtained from the Langostinos y Camarones de Oriente Laboratory, Boca del Río, Veracruz, Mexico. Upon arrival at our facilities, the shrimp were acclimatized for one week in 500 L holding tanks with the conditions prevailing at the bioassay room, before the growth test. Shrimp were individually weighed on a digital scale after blotting off excess water with a wet cloth. Over the first three days after distributing the animals, dead shrimp were replaced by others taken from a pool of animals fed with the same diet. A photoperiod was set in order to provide 12 h of light and 12 h of dark. Shrimp were fed twice daily to achieve an apparent satiation (50% of the portion at 9:00 and 17:00) for 42 days. Any feed remaining in the tank was removed by siphoning before performing the feeding. During the first 2 days, daily feeding was fixed at 10% of the biomass inside the tanks and subsequently adjusted to allow a small excess of remaining feed in each tank until the next feeding. The previously weighed feed strands were fragmented into small pieces for each feeding period in order to ensure a sufficient amount of pellets per shrimp.
Assessment of growth performance parameters. The shrimp in each tank were individually weighed at 7-day intervals in order to assess growth and adjust the feeding ratios. Survival and feed intake were recorded daily and the feeding ratios were adjusted after considering the amount of shrimp per tank and the remaining feed on the latter. The following variables were evaluated: weight gain (%) = [(mean individual final weight (g) - mean individual initial weight (g)) / mean individual initial weight (g)] x 100. Feed intake (g shrimp-1 42 days-1) = (amount of feed provided - remaining feed)/amount of shrimp. Feed conversion ratio = feed intake (g)/live weight gain (g). Survival (%) = (amount of surviving shrimp/initial amount of shrimp) x 100. When the experiment began, a pooled shrimp sample was taken to determine the initial moisture and nitrogen content. After concluding the experiment, five shrimps per tank were randomly sampled to estimate final moisture and nitrogen content. Shrimp samples were freeze-dried, ground in a coffee grinder, and stored until further analysis. Nitrogen retention efficiency (NRE %) was estimated as follows: NRE = [(final mean body weight (g) * final crude protein content on carcass (%)) - (initial mean body weight (g) * initial crude protein content on carcass (%)) / amount of crude protein consumed (g)] * 100.
Statistical analysis. The results are presented as means ± standard deviations. Average body weights per tank were used to calculate growth rates and feed-conversion ratios. Weights, feed intake, growth rate, feed-conversion ratio, survival, and nitrogen retention efficiency were analyzed by a one-way ANOVA comparing the experimental diets and subsequently by a Tukey’s multiple range test (α = 0.05, SSPS 16.0, 2007, SPSS Inc., Chicago, Illinois).
Results
Experimental diets. The chemical composition of all test diets was similar among treatments: crude protein, crude lipids, and ash content (on a dry weight basis) ranged between 39 and 40%, 8.3 and 9.0%, and 8.6 and 9.0%, respectively. Aflatoxin concentration in all contaminated diets was 75 µg kg-1 and the predominant aflatoxin type was B1 (60.7 µg kg-1), followed by B2 (9.7 µg kg-1), and G1 (4.3 µg kg-1). Concen trations of other mycotoxins in all contaminated diets was below the detection limit. In all non-contaminated diets, mycotoxin levels were also below detection limits.
Growth performance parameters. The results obtained after measuring growth, feed intake, and nitrogen-retention efficiency are shown in Figures 1, 2, and 3. The growth rate was similar when diets were compared after 35 days. However, after concluding the experiment, shrimp fed with contaminated diets showed a significantly decreased growth rate (32% less), when compared to those fed with uncontaminated diets. The supplementation of Aflabalan® resulted in similar growth rates in comparison to those measured after supplementing a contaminated diet, whereas Mycosorb® and Mycofix plus® supplemented diets exhibited higher growth rates (24-29%) when compared to shrimp fed with contaminated diets, although they were not statistically different. Shrimp fed with contaminated diets exhibited a significantly decreased feed intake from day 28 to the end of the experiment. After this, the latter parameter decreased by 29%. The supplementation of Aflabalan® and Mycofix plus® to contaminated diets did not counteract the decreased feed intake caused by the presence of aflatoxins. The addition of Mycosorb® to these diets resulted in higher feed intake when compared to those shrimps fed with contaminated diets, although the results were not significantly different. The addition of contaminated grains or aflatoxin binders did not affect the feed-conversion ratio by the end of the experiment. These ranged between 1.2 and 1.3 when the different diets were compared. The survival of shrimp fed with contaminated diets was slightly lower when compared to those fed with the other experimental diets (80 versus 88 - 93%), although differences were not significantly different. The addition of aflatoxin-contaminated corn to the diets decreased nitrogen retention efficiency by 32% and when commercially available aflatoxin commercial binders were tested, they did not improve nitrogen-retention efficiency.
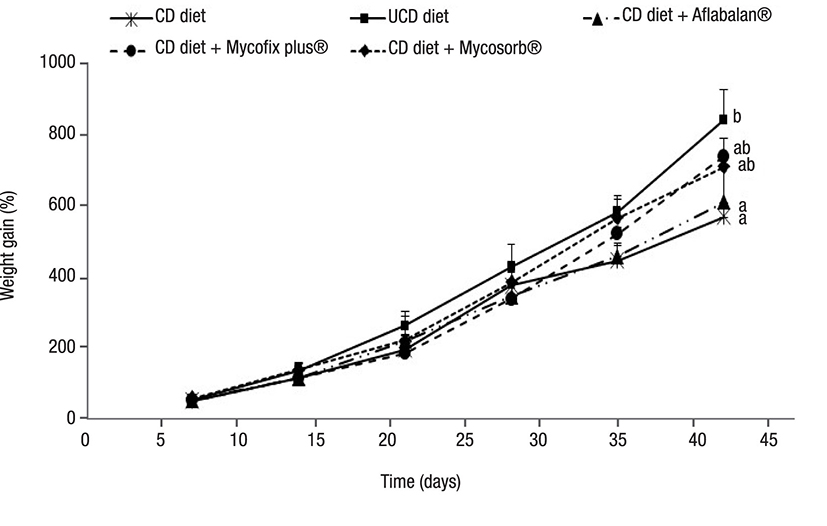
Figure 1 Growth rate in shrimps (Litopenaeus vannamei) fed with aflatoxin-contaminated diets. Each point represents the mean value of four replicates tanks along with its standard deviation. Different letters show significant differences (p < 0.05). CD = contaminated diet, UCD = uncontaminated diet
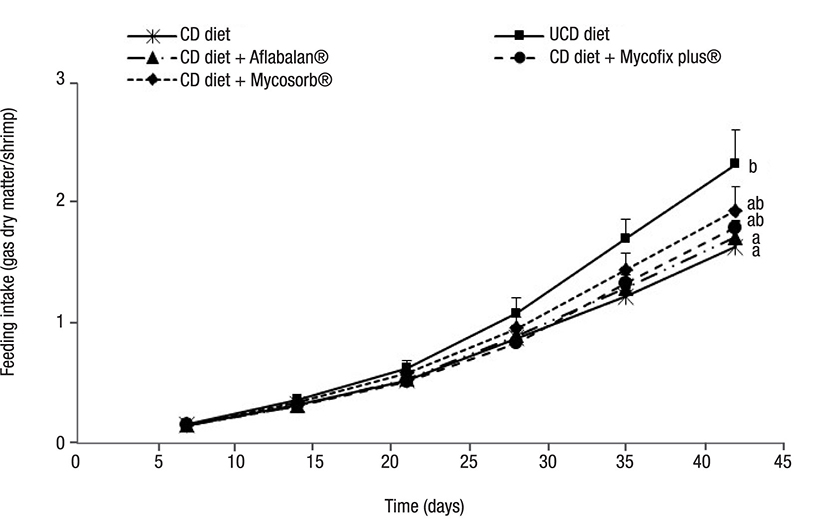
Figure 2 Feed intake in Litopenaeus vannamei juveniles fed with aflatoxin-contaminated diets. Each point represents the mean value of four replicates tanks along with its standard deviation. Different letters show significant differences (p < 0.05). CD = contaminated diet, UCD = uncontaminated diet.
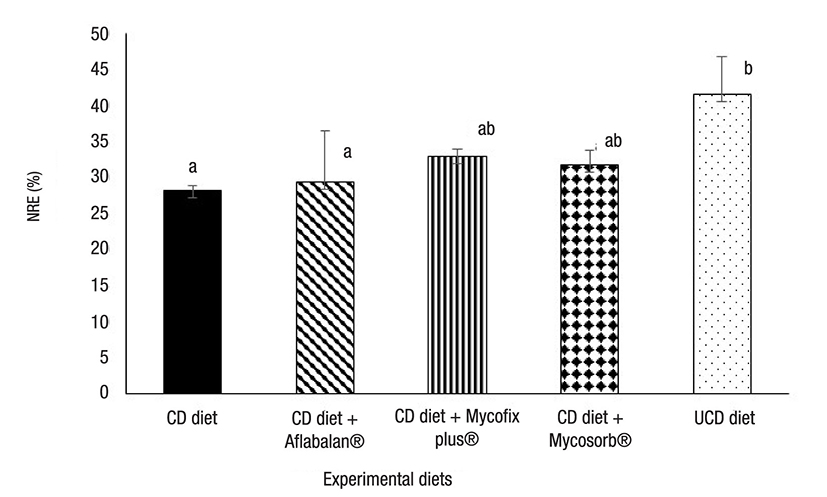
Figure 3 Nitrogen retention efficiency (NRE) in Litopenaeus vannamei juveniles fed with aflatoxin-contaminated diets. Each point represents the mean value of four replicates tanks per treatment. Different letters show significant differences (p < 0.05). CD = contaminated diet, UCD = uncontaminated diet.
Discussion
Dietary aflatoxin supplementation to shrimp. Contamination by aflatoxins of diets manufactured for aquaculture is one of the most important problems currently challenging the shrimp-farming industry caused by increasing levels of plant-derived ingredients in aqua feeds (Santos et al., 2010; Anater et al., 2016). Feeding shrimp with diets contaminated by pure aflatoxin (Suppadit et al., 2006; Arunlertaree et al., 2007; Zeng et al., 2015) or with contaminated grains (Tapia-Salazar et al., 2012; García-Pérez et al., 2013) has resulted in lower feed intake, scarce weight gain, and, occasionally, in mortality and immune system deterioration. The results observed in this study regarding growth rates, feed intake, feed-conversion ratio, nitrogen retention and the survival of shrimp L. vannamei fed with diets containing low aflatoxin levels are in accordance with previously obtained results. Tapia-Salazar et al. (2012) and García-Pérez et al. (2013) point out that a significantly reduced feed intake and weight may occur without affecting survival because of the synergic effect induced by low aflatoxins levels (B1, B2, G1, and G2). In this study, the overall dietary aflatoxin was 75 µg kg-1, and aflatoxin B1 (60.7 µg kg-1) was the predominant type, followed by B2 (9.7 µg kg-1) and G1 (4.3 µg kg-1). The results obtained in this study confirm that a combination of aflatoxins at low levels impairs shrimp’s performance. The lack of effect on the survival rates of shrimp associated with low inclusion levels in their diet is in line with previous results observed for shrimp fed with low levels of pure aflatoxin (Gopinath & Raj, 2009; Zeng et al., 2015) or naturally contaminated grains (Tapia-Salazar et al., 2012; García-Pérez et al., 2013). The background information on the effect of dietary aflatoxin on nitrogen retention in shrimp is scarce. In this study, a significant decrease of nutrient-retention efficiency (30%) was ob served when shrimp were fed with aflatoxin-contaminated diets; this agrees with the results published by Zeng et al. (2015), as they reported a significant decrease of crude protein (approximately 6%) and crude lipids (approximately 27%) in whole L. vannamei carcasses previously fed with increasing levels of pure aflatoxin. Studies have shown that aflatoxin consumption causes hepatotoxicity, inhibits protein synthesis, and causes immunosuppression and metabolic alterations (Monson et al., 2015). This effect is enhanced by a reduced feed intake. The lower nutrient retention observed in shrimp fed with aflatoxin-contaminated diets was probably caused by several complications derived from dys functional metabolic processes during aflatoxicosis.
Efficiency of aflatoxin-binding agents in reducing aflatoxicosis in shrimp. In farm animals, the addition of absorbing or biotransfor ming agents to diets is a strategy designed to counteract the negative effects caused by the consumption of mycotoxins (Surai & Dvorska, 2005; Kolosova, & Stroka, 2011; Gowda et al., 2013; Wielogórska et al., 2016). Some of the commercially available mycotoxin-binding products possess a formulation consisting of multiple sequestering components (antioxidants, probiotics, yeast and/or plants, among others) that con tribute more effectively to decreasing most of the effects caused by mycotoxin consumption, such as decreases of growth, feed intake, the production of reactive oxygen species, lipid peroxidation processes, the impairment of enzymes involved in different metabolic processes (di gestion, immune system etc.) (Surai & Dvorska, 2005; Oguz et al., 2011; Wielogórska et al., 2016). However, their effectiveness depends on se veral factors such as specificity towards some mycotoxins and their concentration levels, their presence as a pure compound or as a mix ture, pH, the clay source, absorption/desorption ability, etc. (Devreese et al., 2013; De-Mil et al., 2015; Wielogórska et al., 2016). Regarding terrestrial animals, products are already available for preventing or co rrecting the negative effects caused by mycotoxin-contaminated feed when suitable levels are used (Surai & Dvorska, 2005; Boudergue et al., 2009; Kolosova & Stroka, 2011; Gowda et al., 2013; Wielogórska et al., 2016). One of the problems faced by the aquaculture industry re garding the effectiveness of commercial products intended to decrease aflatoxicosis in aquatic organisms is the limited amount of available information. Often the inclusion levels used for testing are those ob tained for other species. In this study, as no information on Aflabalan® was available for other species, the inclusion levels consisted of a 2 g kg-1 preventive dose based on the manufacturer’s recommendations. A 2 g kg-1 corrective dose of Mycosorb® was used because inadequate results were observed on terrestrial animals when it was included in aflatoxin-contaminated diets at levels ranging between 0.5 and 1.5 g kg-1. Finally, a 2.5 g kg-1 level of Mycofix plus® was selected as op timal results were observed on terrestrial animals fed with aflatoxin-contaminated diets. The results obtained in this study showed that after feeding shrimp with an aflatoxin-contaminated diet supplemented with 2 g kg -1 Aflabalan®, similar growth rates were obtained in comparison to those fed with non-contaminated diets (control diets). The addition of Mycosorb® and Mycofix-plus® slightly improved these parameters, although they were not significantly similar when compared to shrimp fed with non-contaminated diets. After comparing the ingredients of all test products, it was noted that Mycosorb® contains glucomannan cell walls from S. cerevisiae and cell walls from microalga, whereas Mycofix plus® contains bentonite, plant extracts, and some yeast or bacterial species purportedly useful for detoxifying several mycotoxins. Studies on broilers and rats have shown that some of these additives reverted some of the negative effects caused by the presence of mycotoxins (Po litis et al., 2005; Abdel-Wahhab et al., 2006; Manoharan et al., 2008). Despite the fact that positive results were obtained by including these additives on other species fed with mycotoxin-contaminated feed, this was not the case for shrimp. The different results may be explained by several factors such as the respective supplemented levels. More studies are needed in order to test higher levels that may counteract the effects of aflatoxicosis in shrimp.
We conclude that after feeding a juvenile population of the Paci fic white shrimp L. vannamei diets that contain low aflatoxin levels, they had a significantly reduced growth rate, feed intake, and nitrogen-retention efficiency, but their survival and feed conversion ratio were not affected. Feeding juvenile L. vannamei with aflatoxin-contaminated diets supplemented with Aflabalan®, Mycosorb® and Mycofix plus® at any of the levels we tested in this study did not reproduce the growth performance observed on shrimp fed with non-contaminated diets.











 text new page (beta)
text new page (beta)


