Introduction
Research has established that organisms inhabiting marine habitats polluted by heavy metals, polycyclic aromatic hydrocarbons, polychlo rinated biphenyls, etc., may experience adverse physiological effects (Ruiz et al., 2014). Biological or somatic indices and the weight-length relationship are among the tools most commonly used to assess the overall health (nutritional status and energy reserves) and physiological status (growth, reproduction, etc.) of organisms, since they provide information on the overall effects on biota of stress, environmental factors, and pollution (Lucas & Beninger, 1985; Filgueira et al., 2013). Some advantages of these indicators include low cost and prompt results. These measurements are representative and sensitive to environmental changes, hence they are very useful in obtaining a preliminary diagnosis of the physiological status of organisms living in polluted areas (Mercado-Silva, 2005).
The port of Santa Rosalía is located within the Gulf of California in the central eastern coast of the Baja California peninsula Mexico. It is characterized by high concentrations of heavy metals in sediments and soils associated with copper mining and smelting that has occurred there for nearly a century (Wilson & Rocha, 1955; Huerta-Diaz et al., 2014). As a result, coastal marine sediments near this port have abnormally high levels of some heavy metals, particularly Cu (3,390 mg kg-1), Zn (1,916 mg kg -1 ), Co (166 mg kg -1 ), Mn (6,770 mg kg -1 ), Pb (226 mg kg -1 ), and U (11.8 mg kg -1 ), and are potentially toxic to the marine biota (Shumilin et al., 2013). In addition, this port is located in a delta of streams so freshwater discharges are common during the rainy season and include urban wastewater that adds mostly organic pollutants to the coastal zone (Huerta-Diaz et al., 2014).
The chocolate clam, Megapitaria squalida (Sowerby, 1835), is one of the most abundant bivalve species in Baja California Sur, and in the past few years it has become an alternative resource when other spe cies of higher market value are not available due to fishing restrictions (closed season). More recently, however, this clam is being harvested throughout the year due to its local and regional importance, thus be coming a fishery resource of great importance (Arellano-Martínez et al., 2006). M. squalida can be considered a good indicator of environmen tal health, given its ability to concentrate heavy metals, its widespread abundance in the region, and its sedentary nature. It should, therefore, provide a comprehensive picture of the health of its ecosystem (Méndez et al., 2006; Frías-Espericueta et al., 2008; Cantú-Medellín et al., 2009).
The present study evaluates the health status of M. squalida inha biting the coastal zone of the Santa Rosalía mining port, Gulf of Califor nia, Mexico, through the analysis of size, condition index, and weight-length relationships. Additionally, these results were compared with data of clams from four coastal areas of the Baja California peninsula deemed pristine. The main objective was to determine whether clams in the mining region show adverse effects because of the contamination.
Materials and methods
Sampling. Monthly sampling (30 individuals on average) was done from May 2012 to April 2013 in a marine area adjacent to the“hot spot” (area with high concentrations of heavy metals in sediments) of the port of Santa Rosalía, Gulf of California (27°20’ N and 112°16’ W) (Shumilin et al., 2000; Shumilin et al., 2013). Samples were also collected from San Lucas, a site located 13 km south of Santa Rosalía, as well as from Bahía de La Paz, Laguna Guerrero Negro, and Bahía Magdalena (Figure 1). Since no mining associated with heavy metals is conducted in the latter, they are considered pristine or low-impacted areas (Shumilin et al., 2000; Cadena-Cárdenas et al., 2009). For each specimen, shell length (maximum distance along the anterior-posterior axis) (± 0.1 mm), total weight, wet weight (off-shell weight), and shell weight (± 0.1 g) were recorded.
Figure 1 Geographical location of the study areas in localities of the northern portion of the Mexican Pacific.
Size frequency, condition index, and weight-length relationships. To analyze the size distribution of M. squalida for each zone, frequency histograms for shell length were constructed. The physiological status was estimated by calculating the condition index as the relative (percentage) relationship between wet weight (no shell) and total weight (Mouneyrac et al., 2008). As additional indicator of health status, growth was examined by considering the weight (total weight, wet weight or shell weight) of each specimen with respect to length (Tlili et al., 2011). To this end, the relationship between weight and shell length was calculated using the potential function y = ax b , where: y is total weight, wet weight or shell weight, a and b are constants, and x is length. The value of b is the coefficient of allometry, used as an indicator of the type of growth exhibited by a given species (Gaspar et al., 2001). For all relationships, we calculated the coefficient of determination (R 2 ) to determine the degree of association between weight and length.
Statistical Analysis. To test for significant differences in size, weight, and condition index between specimens from different areas of stu dy, an analysis of variance (ANOVA) was used, followed by Tukey’s test when significant differences were found. Because the condition index values are percentages, these were normalized through an arc sine transformation (Zar, 1996). To determine whether the growth of M. squalida is isometric (b = 3; increase in the same proportion in weight and height), negative allometric (b < 3, greater increase in size vs. weight), or positive allometric (b > 3, greater increase in weight vs. size), a Student’s t test was performed (H0, b = 3) (Ricker, 1975; Zar, 1996). In addition, the growth type of M. squalida was compared bet ween sites through a residual sum of squares (Ratkowsky’s ARSS) for the comparison of slopes in nonlinear functions (Chen et al., 1992). This test assesses statistical differences between two or more curves by calculating an F value. Statistical testing was performed with the soft ware STATISTICA for Windows (version 10, Statsoft), with a significance level of α = 0.05 for all tests.
Results
A total of 1,687 specimens were analyzed: 370 clams from Santa Rosalía, 326 from San Lucas, 305 from Bahía de La Paz, 333 from Laguna Guerrero Negro, and 353 from Bahía Magdalena.
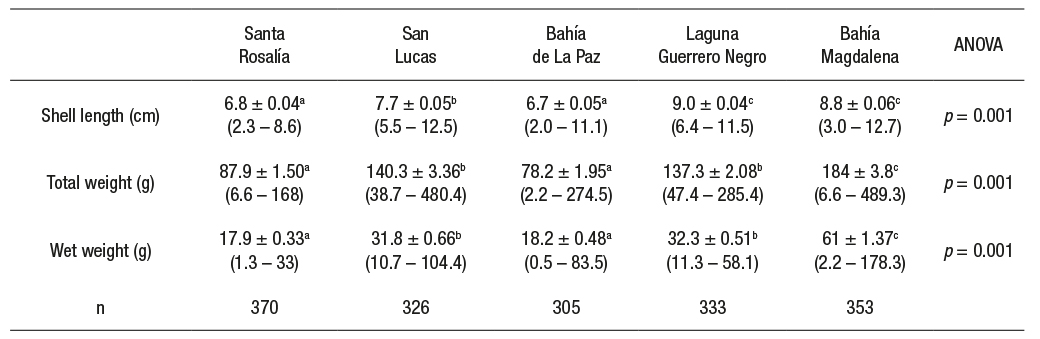
Biometrics. Significant differences were found between study areas in all biometric parameters of M. squalida (ANOVA, p < 0.05) (Table 1). The largest specimens in length were observed in Laguna Guerrero Negro and Bahía Magdalena (F(4, 1687) = 385.6, p < 0.05), followed by San Lucas. The smallest clams were collected in Santa Rosalía and Bahía de La Paz. The heaviest clams were found in Bahía Magdalena (F(4, 1687) = 235, p < 0.05), followed by San Lucas and Laguna Guerrero Negro. The lightest clams were collected in Santa Rosalía and Bahía de La Paz. The highest wet weight (F(4, 1687) = 471.2, p < 0.05) was recorded in Bahía Magdalena, followed by Laguna Guerrero Negro and San Lucas. The lowest wet weight values were recorded in Bahía de La Paz and Santa Rosalía.
Table 1 Biometric parameters of Megapitaria squalida by sampling site in localities of the northern portion of the Mexican Pacific. Mean ± standard error (minimum - maximum). Means with a different letter indicate significant differences.
Size frequencies. The size-frequency distribution of M. squalida by sampling site is shown in Figure 2. Three groups were identified: small (< 7 cm), medium (8 cm), and large (> 9 cm) clams. Large clams occurred more frequently in Laguna Guerrero Negro and Bahía Magdalena, followed by San Lucas. Santa Rosalía and Bahía de La Paz had smaller clams when compared to all other sites.
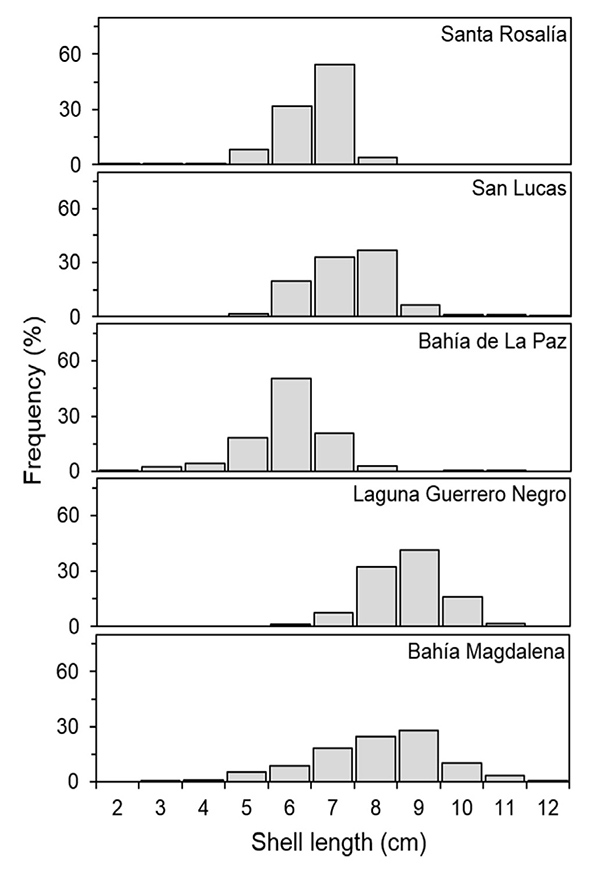
Figure 2 Size-frequency distribution (shell length) of Megapitaria squalida by sampling site in localities of the northern portion of the Mexican Pacific.
Condition Index. The variation in the condition index of M. squalida between sites is shown in Figure 3. Significant differences were found in the condition index between sites (F(4, 1687) = 829.1, p < 0.05). Clams with a significantly higher index were found at Bahía Magdalena (33%), followed by Bahía de la Paz (25.8%). Clams from San Lucas and Lagu na Guerrero Negro showed intermediate condition index values (23.6% and 23.5%, respectively), while clams from Santa Rosalía showed a significantly lower index (19.9%) than all other sites.
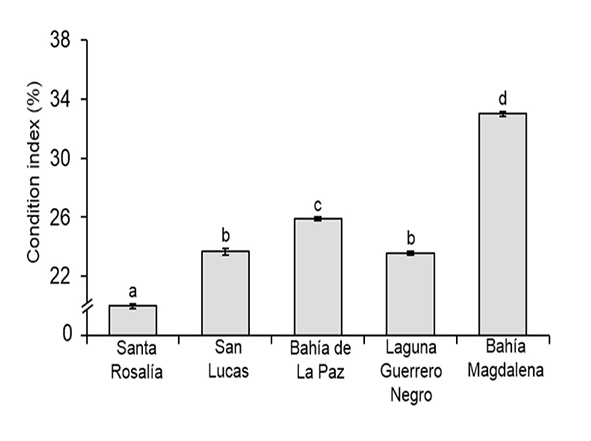
Figure 3 Variation in the condition index of Megapitaria squalida between sam pling sites in localities of the northern portion of the Mexican Pacific. Means (± standard error) with a different letter indicate a significant difference (ANOVA and Tukey’s test, p < 0.05).
Weight-Length relationships. Weight-length relationships (total weight-shell length, wet weight-total length, and shell weight-total length) of M. squalida by site are shown in Figure 4. All relationships fit the potential function (y = axb), with coefficients of determination (R2) between 0.73 and 0.95 and a significance of p = 0.001. In general, the coefficients of allometry (b) fluctuated between 2.35 and 3.32 across sites (Table 2). Ratkowsky’s ARSS revealed significant differences between the slopes of each relationship analyzed among sites (p = 0.001). Compared to the other areas where larger and heavier clams were recorded and growth was mainly isometric, Santa Rosalía clams gained little weight (total, wet, or shell) as shell length increases.
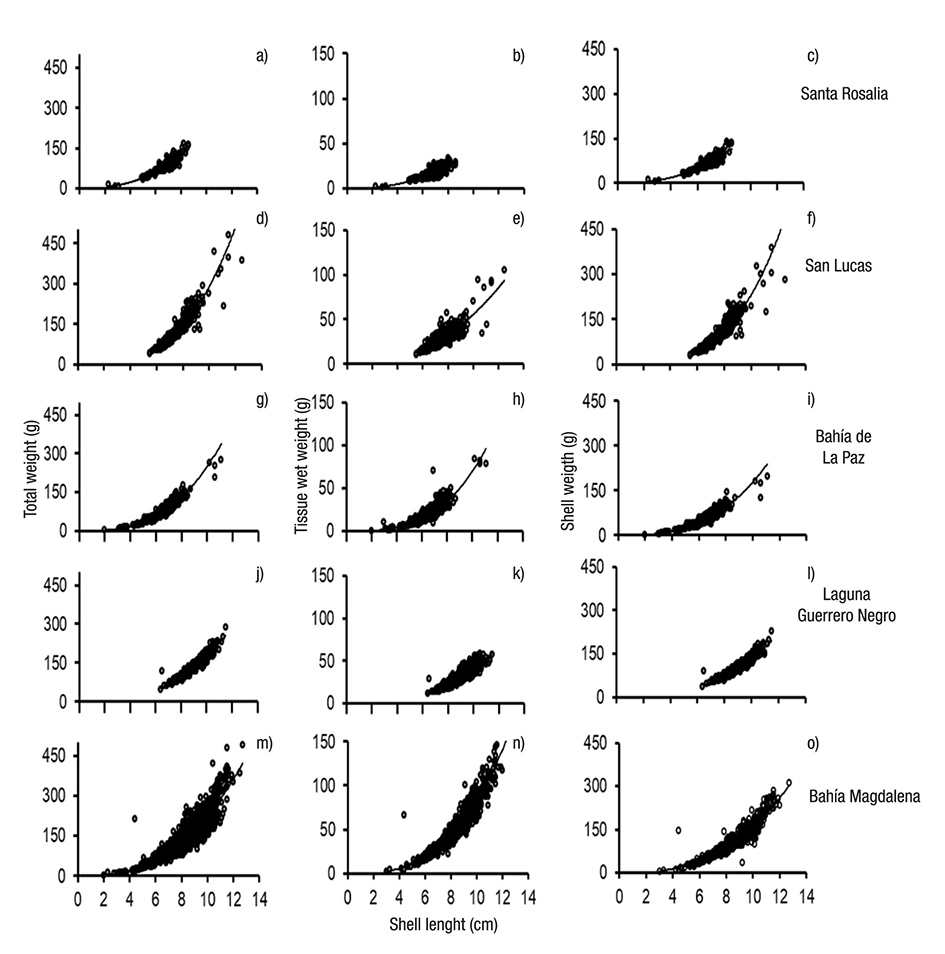
Figures 4 a-o. Weight-lenght relationships of Megapitaria squalida by sampling sites in localities of the northern portion of the Mexican Pacific. a-c) Santa Rosalía. d-f) San Lucas. g-i) Bahía de la Paz. j-l) Laguna Guerrero Negro. m-o) Bahía Magdalena. a, d, g, j, m) Total weight-length. b, e, h, k, n) Wet weight-length. c, f, i, l, o) Shell weight-length.
Discussion
Our results revealed the existence of three groups of clams based on the differences in the variables analyzed (shell length, total weight, wet weight, and condition index). The Bahía Magdalena group had the largest clams with the best condition. This area is a Biological Activity Center (BAC) characterized by high productivity throughout the year (Lluch-Belda et al., 2000), resulting in abundant food availability for suspension feeders and conditions that favor better growth and condi tion for M. squalida. The second group includes clams of intermediate size from San Lucas and Laguna Guerrero Negro; although clams from San Lucas displayed a smaller shell length compared to specimens from Laguna Guerrero Negro, clams from these two areas shared a similar total weight, wet weight, and condition. The third group comprises clams from Bahía de La Paz and Santa Rosalía, which were the smallest clams in terms of length, total weight, and wet weight. The small size of clams from Bahía de La Paz may be related to the intense fishing in this area, as M. squalida has been considered a resource at its peak capacity (López-Rocha et al., 2010); in contrast, Santa Rosalía clams are not an appealing resource for local fishers and, nonetheless, clams are small.
Although the fishing intensity of a resource and the environmental conditions in each area influence the biological characteristics and po pulation structure of a species, results from this study suggest that the biometric differences of the Santa Rosalía clams were likely not entirely attributable to these factors. The maximum shell length of Santa Rosalía clams did not exceed 8 cm, despite this clam population not being commercially exploited, while clams from other areas reached sizes between 11 and 12 cm (including those from Bahía de La Paz), i.e., the largest recorded sizes across the Baja California Sur coast (Singh et al., 1991). In addition to the small size, Santa Rosalía clams showed the lowest condition index values, and although clams from this area and from Bahía de La Paz were of similar size, the condition of animals from the former site was significantly poorer. Condition index is affected by several factors, such as seasonal changes in food availability and/or quality in each site (Boscolo et al., 2003; Nicholson & Lam, 2005). In this regard, Santa Rosalía is deemed a nutrient-poor water body of low primary productivity; although upwelling events occur, they are weak because of the stratification of the water column (Santamaría-del Ángel et al., 1999). This situation could explain the poor condition and small sizes of clams in this area. This explanation was ruled out, however, because in San Lucas, an area located just 13 km south of Santa Rosalía, clams displayed a better condition and were larger despite sharing similar food availability and water temperatures with the port of Santa Rosalía (3.0 mg·m-3 chlorophyll a and 23.5 °C for San Lucas; 2.9 mg·m-3 chlorophyll a and 23.5 °C for Santa Rosalía, averages for 2011 to 2013 obtained from the NOAA Coastal Zone Color Scanner).
The coastal sediments near the Santa Rosalía mining port contain heavy metals that are bioaccumulated by organisms (Shumilin et al., 2011), as reported for brown seaweed Padina durvillaei Bory Saint-Vin cent, 1827 (Rodríguez-Figueroa et al., 2009) and for mussels Modiolus capax (Conrad, 1837) (Gutiérrez-Galindo et al.,1999) and Mytilus edulis Linnaeus, 1758 (Cadena-Cárdenas et al., 2009). The chocolate clam, M. squalida, feeds by filtering organic matter suspended in the water column (mainly phytoplankton), so it is likely that it bioaccumulates me tals, as documented for this species elsewhere (Méndez et al., 2006). Although this study did not determine the concentration of heavy me tals in clam tissues, it has been reported that abnormal concentrations of these elements in surface sediments can cause negative biological effects in up to 50% of the marine organisms inhabiting this area (Long et al., 1995; Shumilin et al., 2011). Bivalves mollusks living in polluted areas or that are exposed to high pollutant concentrations usually show lower growth rates - and therefore a smaller size - in addition to a poor condition. This occurs because energy reserves (carbohydra tes, lipids, and proteins) are allocated to depurating pollutants from the body at the expense of the other physiological demands (Leung & Furness, 2001; Nicholson & Lam, 2005; Peteiro et al., 2006). Differen ces in size and condition between clams from Santa Rosalía and those living in other areas are likely a consequence of impaired growth rates induced by high concentrations of heavy metals, since clams may be allocating energy reserves to detoxification at the expense of growth, hence affecting the overall condition of these organisms (Lucas & Be ninger, 1985; Nicholson & Lam, 2005). The relationship between high concentrations of heavy metals and a poor condition has been widely reported for various bivalve species such as the clams Macoma balthica (Linnaeus, 1758) and Cerastoderma edule (Linnaeus, 1758), and the mussels M. edulis and Perna viridis (Linnaeus, 1758) (Hummel et al., 1997; Nicholson, 1999). Similarly, the mussel Mytilus galloprovincialis Lamarck, 1819 and the venerid Meretrix meretrix (Linnaeus, 1758) from polluted areas (Hg, As, Cu, Pb, Zn, Cd, and Cr, as well as polychlorinated biphenyls) showed low condition indices versus specimens from less polluted areas (Pampanin et al., 2005, Meng et al., 2013).
The analysis of the weight-length relationships revealed that in San Lucas, Bahía de La Paz, Laguna Guerrero Negro, and Bahía Mag dalena, M. squalida showed isometric growth. This is the most common type of growth in marine bivalves, and is generally influenced by chan ges in environmental variables (Gaspar et al., 2001). In contrast, clams from Santa Rosalía displayed negative allometric growth, i.e., little in crease in weight as shell length increases, which suggests physiologi cal impairment due to environmental stress (Malathi & Thippeswamy, 2011). Negative allometric growth is frequently attributed to elevated environmental pollutants, as reported for the mussel M. galloprovincia lis in the coastal area of Galicia in northwestern Spain (Peteiro et al., 2006), and for Donax trunculus Linnaeus, 1758 in the Gulf of Tunis (Tlili et al., 2011). In conclusion, it is clear that the biometric parameters, the condition index, and the growth type of clams that inhabit the Santa Rosalía port area in the Gulf of California all differ from the pattern recorded for other areas. These other areas include San Lucas, a site located a few kilometers away from the pollution hot-spot and where the conditions of temperature and food availability are similar to those in the port area. Based on these findings, we conclude that M. squalida displays poor health and growth in the port of Santa Rosalía area, which are most likely caused by the high levels of heavy metals in sediments coupled with pollutants from wastewater discharges from the urban sewerage system. Further studies on the concentrations of metals in clam tissues and their potential risks to human and wildlife consumers are warranted.











 text new page (beta)
text new page (beta)



